Session Information
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Biomechanics plays an important role in knee osteoarthritis (OA). A new biomechanical footwear system aims at altering knee loading patterns and retraining neuromuscular control of the lower extremities. It consists of shoes with two adjustable convex pods at the soles, which are adjusted based on gait analysis, with the hypothesis that adjustments of the location of the pods will alter limb biomechanics so as to unload diseased compartments of the knee and that walking on the convex pods will facilitate muscular retraining.
The aim was to compare the efficacy and safety of the new biomechanical footwear with an identical appearing shoe with flat pods (the sham device) in relieving pain and improving physical function in patients with knee OA.
Methods: In this randomized sham-controlled trial, patients with radiological knee OA (K/L grade ≥2) and moderate pain on the WOMAC pain subscale (≥3 on a standardized scale from 0 to 10) were randomly assigned 1:1 to the biomechanical footwear or the sham device. The same shoe was provided for bilateral use. Patients in both groups were instructed to use the footwear for 30 minutes/day during the first week, and to increase use by 10 minutes/day each week to a maximum of 5 hours/day at 24 weeks. After 4, 8, 12, and 16 weeks, each patient’s footwear was re-calibrated by technicians. Because the sham device had no adjustable pods on the soles, technicians pretended to make appropriate changes.
The primary endpoint was knee pain at 24 weeks in the knee with more pain at screening, assessed with the WOMAC pain subscale. Secondary outcomes were WOMAC physical function and stiffness subscales. These outcomes were analyzed using linear models adjusted for baseline values and the two stratification factors uni- vs. bilateral, and medial vs. lateral osteoarthritis at randomization, using multiple imputation.
Results: Of 697 patients assessed for eligibility, 220 were randomized: 111 to the experimental footwear and 109 to the sham device. The mean age was 65.2 years (SD 9.2) and the mean body mass index was 28.0 (SD 4.6). Overall, 47.3% were women and 88.2% had medial knee OA in the index knee. The mean WOMAC pain score at baseline was 4.1 (SD 1.9). Seven patients in the experimental group and 13 in the sham group dropped out. At the end of the trial, the adjusted mean difference for WOMAC pain was 1.34 (95% CI 0.92 to 1.77) in favor of the experimental footwear. The adjusted mean difference was 1.42 (0.93 to 1.91) for WOMAC stiffness and 1.12 (0.73 to 1.50) for WOMAC physical function (Figure 1). Three serious adverse events occurred in the experimental group, compared with 9 in the sham group; none were treatment-related. Thirty adverse events occurred in the experimental group, compared with 36 in the sham group; 18 and 17 of these, respectively, were possibly treatment-related.
Conclusion: This trial suggests that the new biomechanical footwear system is both efficacious and safe in relieving knee pain in patients with knee OA.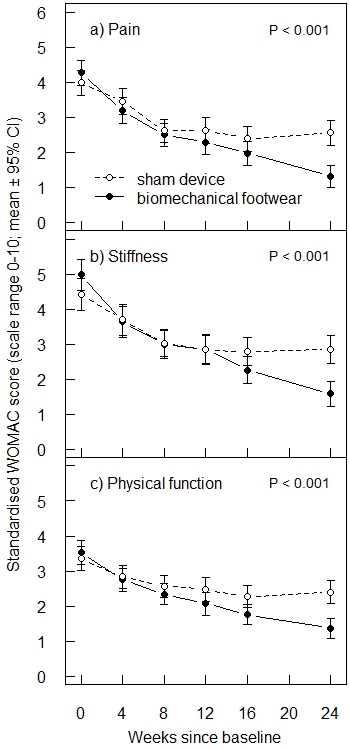
To cite this abstract in AMA style:
Reichenbach S, Heldner S, Lenz A, Felson DT, Jüni P. Biomechanical Therapy for Osteoarthritis of the Knee: A Randomized Controlled Trial [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/biomechanical-therapy-for-osteoarthritis-of-the-knee-a-randomized-controlled-trial/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/biomechanical-therapy-for-osteoarthritis-of-the-knee-a-randomized-controlled-trial/
