Session Information
Date: Sunday, November 5, 2017
Title: Spondyloarthropathies and Psoriatic Arthritis – Pathogenesis, Etiology Poster I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose:
Apremilast (Otezla) is a PDE4 inhibitor approved for the treatment of psoriasis and psoriatic arthritis, but the mechanisms of action of apremilast are not fully described. The objective of this study was to study the downstream effects of apremilast on cells of the inflamed joint in ex vivo models of immune mediated inflammatory arthritis. Therefore, we tested the ex vivo effect of apremilast on the secretion of several cytokines, chemokines and growth factors by synovial fluid mononuclear cells (SFMCs), fibroblast-like synovial cells (FLSs), osteoclasts, synovial macrophages, and osteoblasts.
Methods:
SFMCs and FLSs were obtained from a study population consisting of patients with active RA, or peripheral SpA with at least one swollen joint (for obtaining synovial fluid) (n=14). Peripheral blood mononuclear cells (PBMCs) were obtained from healthy controls and osteoblasts were purchased. SFMCs were cultured for 48 hours with and without addition of apremilast measuring the secretion of a large panel of cytokines, chemokines and growth factors by the Olink proseek multiplex interferon panel and commercially available ELISA assays. These effects were compared with the effects of the TNFα inhibitor adalimumab. Further, FLS-PBMC co-cultures were used to study the secretion of metalloproteinases, SFMCs cultured for 21 days were used to study inflammatory osteoclastogenesis and macrophage differentiation, and a mineralization assay was used to study new bone formation.
Results :
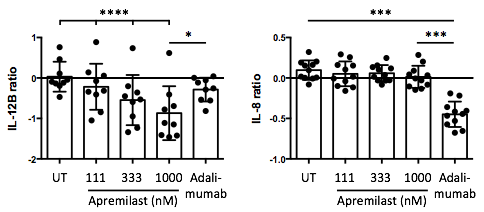
In SFMCs cultured for 48 hours, apremilast decreased the production of IL-12B (P<0.00001) CSF1 (P=0.009), sCD6 (P=0.03), sCD40 (P=0.04), and MCP-1 (P=0.02), and increased the production of CXCL5 (P=0.003) dose-dependently. In sub-analyses, the apremilast induced decrease in cytokine production was greater in cultures with a high lymphocyte count and in cultures from patients with a low C-reactive protein level. Further, apremilast had a very different response signature compared with adalimumab, e.g. with a more robust inhibition of IL-12B (P=0.01) and less inhibition of IL-8 (P=0.0001) (see figure). In FLS-PBMC co-cultures, apremilast decreased MMP3 production (P=0.009). In SFMCs cultured for 21 days, apremilast did not significantly decrease inflammatory osteoclastogenesis (P=0.2). However, apremilast increased the secretion of IL-10 (P=0.04) without affecting the secretion of MCP-1 (P=0.5). Finally, apremilast did not significantly decrease mineralization by human osteoblasts (P=0.2).
Conclusion :
This study reveals the downstream effects of apremilast in ex vivo models of arthritis with a strong inhibition of IL-12B. Further, apremilast induced IL-10 production in synovial macrophages and decreased MMP3 in FLS-PBMC co-cultures. Our findings could explain some of the efficacy of apremilast seen in IL-12/IL-23 driven immune mediated inflammatory diseases such as psoriasis and psoriatic arthritis.
To cite this abstract in AMA style:
Kragstrup TW, Lomholt S, Nielsen MA, Heftdal LD, Schafer PH, Deleuran B. Downstream Effects of Apremilast in Human Arthritic Ex Vivo Models [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/downstream-effects-of-apremilast-in-human-arthritic-ex-vivo-models/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/downstream-effects-of-apremilast-in-human-arthritic-ex-vivo-models/