Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Bone erosion is a common complication of tophaceous gout. Disordered osteoclast activity has been implicated in the pathogenesis of gouty bone erosion. We sought to determine if the addition of denosumab to intensive urate-lowering therapy (ULT) improves gouty bone erosion through targeting receptor activator of nuclear factor kappa-B ligand (RANKL).
Methods: Open-label, parallel-group pilot randomized controlled trial in which 20 participants with gout with at least one confirmed conventional radiographic foot bone erosion were assigned in a 1:1 allocation to receive denosumab (60 mg subcutaneous every 6 months) added to intensive ULT (serum urate ≤5 mg/dL or 300 µmol/L at the time of randomization and continued for the duration of the study) or intensive ULT alone. The primary outcome was the change in the foot computed tomography (CT) bone erosion score from baseline to 12 months. Erosion score was assessed by an experienced musculoskeletal radiologist blinded to study assignment. Secondary outcomes included change in serum c-terminal telopeptide (CTX), functional status by Health Assessment Questionnaire-II, physical and mental health by Short Form Health Survey 12 and pain scores by visual analogue scale (VAS).
Results: The mean age of all study participants was 65.0 (SD 12.4) years and 19/20 were men. Mean disease duration was 15 (SD 9.3) years, serum urate at randomization was 4.3 (0.6) mg/dL and the mean CT erosion score was 6.8 (SD 5.1). Baseline characteristics were balanced between the denosumab and active comparator groups (Table 1). There was no interval change in CT erosion score in either the denosumab or active comparator group after one year of follow-up (Table 2). Serum CTX declined markedly in the denosumab group compared with the active comparator group. Other secondary outcomes did not change between groups (Table 2). One patient developed atrial fibrillation (on denosumab) and another atrial flutter (on active comparator). There were no serious adverse events and no events led to study discontinuation.
Conclusion: In this one-year pilot study, denosumab did not offer additional benefit to intensive urate lowering therapy for gouty bone erosion.
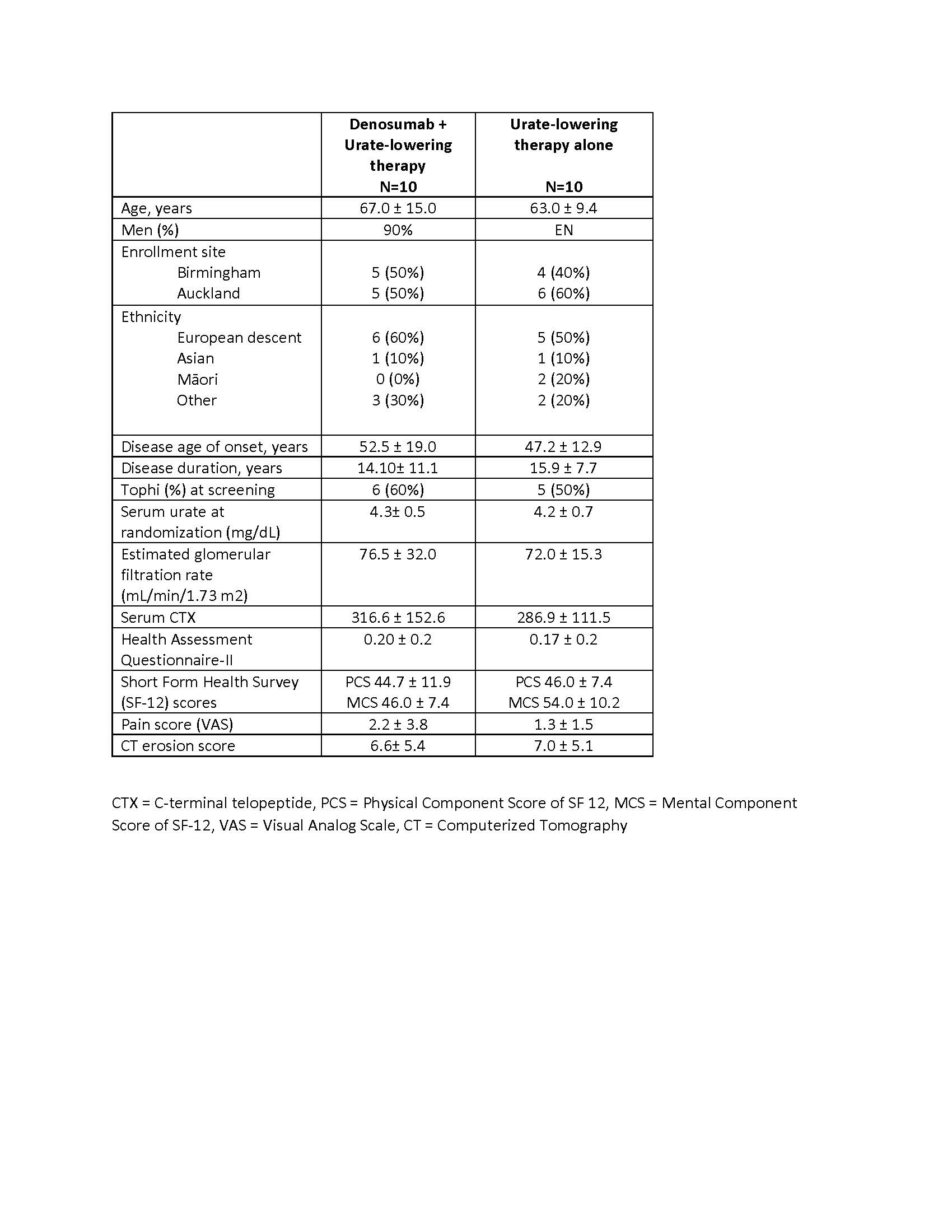 TABLE 1. Baseline characteristics of participants. Unless stated, data are shown as mean ± SD.
TABLE 1. Baseline characteristics of participants. Unless stated, data are shown as mean ± SD.
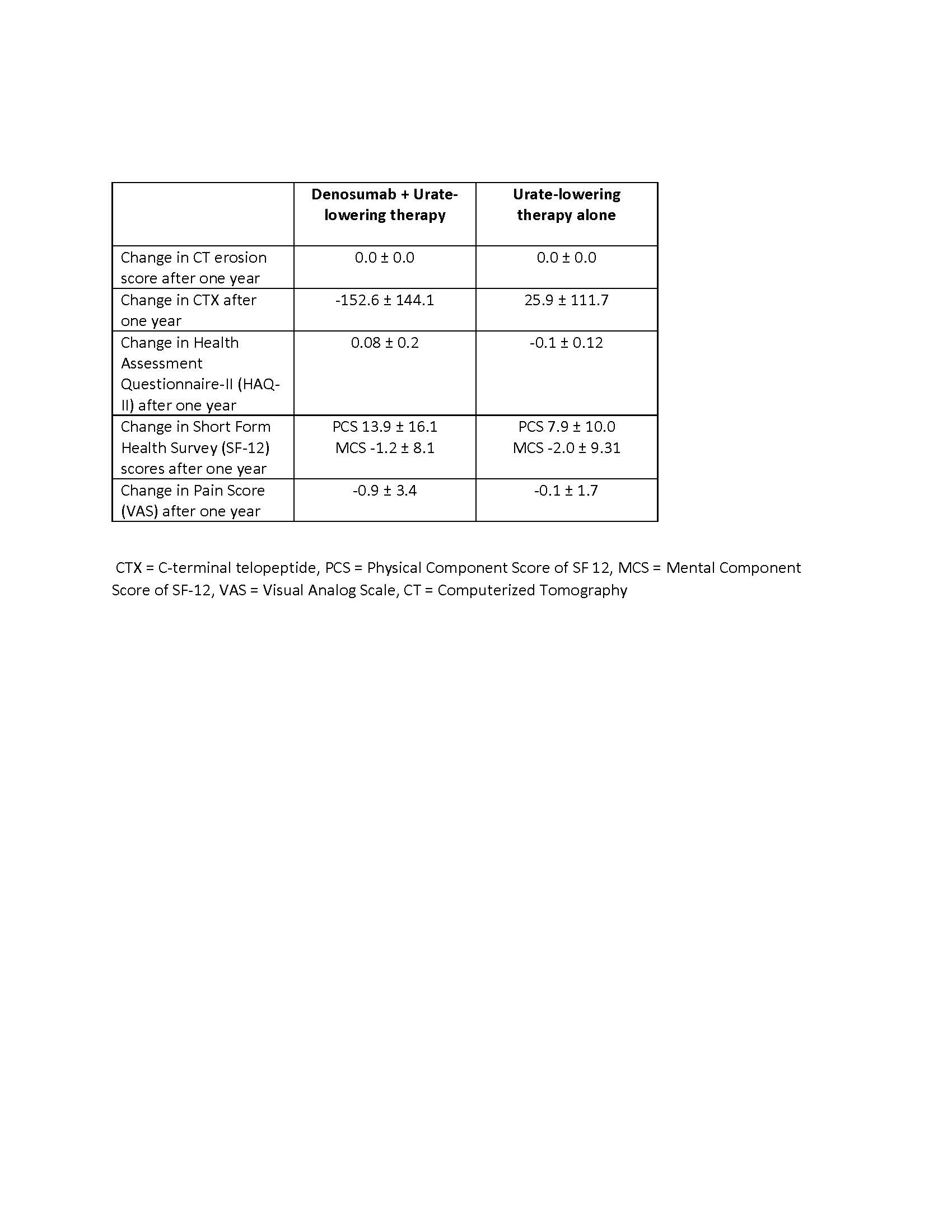 TABLE 2. Primary and Secondary Study Endpoints. Unless stated, data are shown as mean ± SD.
TABLE 2. Primary and Secondary Study Endpoints. Unless stated, data are shown as mean ± SD.
To cite this abstract in AMA style:
Gaffo A, Saag K, Doyle A, Melnick J, Horne A, Foster J, Mudano A, Biggers S, Redden D, Dalbeth N. Denosumab Did Not Improve Computerized Tomography Erosion Scores When Added to Intensive Urate-Lowering Therapy in Gout: Results from a Pilot Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/denosumab-did-not-improve-computerized-tomography-erosion-scores-when-added-to-intensive-urate-lowering-therapy-in-gout-results-from-a-pilot-study/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/denosumab-did-not-improve-computerized-tomography-erosion-scores-when-added-to-intensive-urate-lowering-therapy-in-gout-results-from-a-pilot-study/
