Session Information
Date: Sunday, November 7, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster I: Axial Spondyloarthritis (0908–0939)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Ankylosing spondylitis (AS) – also referred to as radiographic axial spondyloarthritis – is a chronic inflammatory disease that affects the sacroiliac joints and spine. Inhibition of Janus kinase 1 (JAK1) has the potential to simultaneously block multiple inflammatory pathways and lessen disease severity. In the recently completed TORTUGA study, filgotinib (FIL), a preferentially selective JAK1 inhibitor, significantly reduced AS disease activity compared with placebo (PBO).1 The current study was conducted to evaluate the impact of FIL on transcriptional biomarkers in adult patients with active AS from the TORTUGA study.
Methods: TORTUGA (Clinicaltrials.gov identifier: NCT03117270) is a phase 2 double-blind, PBO-controlled study in which 116 AS patients with an inadequate response to >1 NSAID were randomized 1:1 to receive FIL 200 mg or PBO orally once daily for 12 weeks. Whole blood samples from patients were collected in PAXgene tubes at baseline and weeks 1, 4 and 12. Illumina TruSeq Stranded mRNA (50M 100bp PE) was generated for 414 samples from 96 patients, FIL 200 (n=49) and PBO (n=47). Gene-level quantification of RNA-seq counts and transcripts/million (TPMs) was conducted using Salmon (v0.8.2, and gencode GRCh38.p7 v25). Pathway analysis was performed using single sample gene set enrichment analysis (ssGSEA) based on Hallmark 50 pathways from the MSigDB. Treatment effect was evaluated using differential expression analysis by limma.
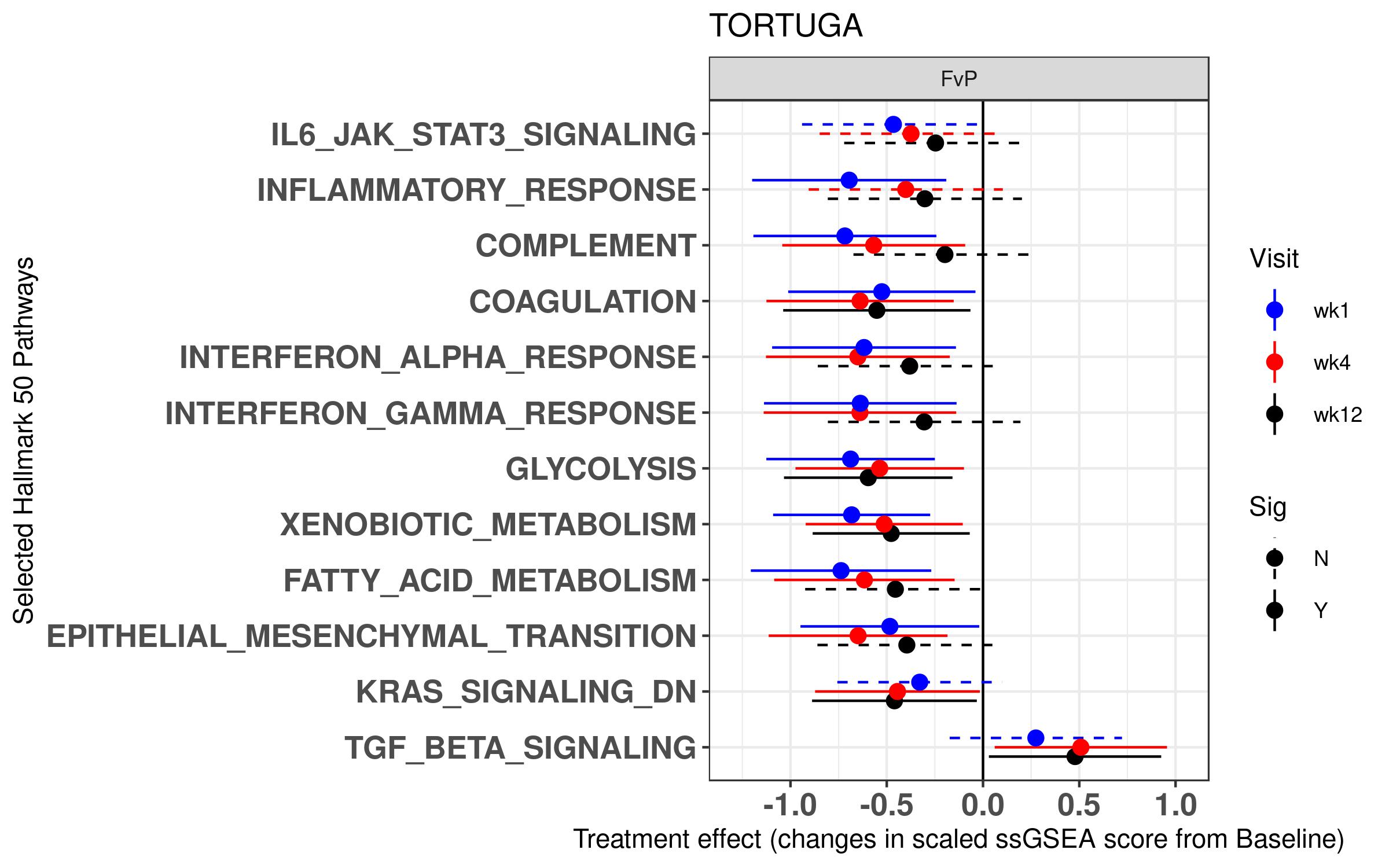
Results: The JAK-STAT, Inflammatory Response, Complement and Coagulation Hallmark 50 pathways were significantly correlated with the C-reactive protein (CRP) levels at baseline, whereas ASDAS correlated with Inflammatory Response and Coagulation pathways, among others. Treatment with filgotinib rapidly decreased several immune pathways at week 1 (Coagulation, Inflammatory Response Complement, Interferon), metabolic pathways (Glycolysis, Fatty Acid Metabolism) and increased the TGF-β Signaling pathway at weeks 4 and 12 (Figure 1). At the gene level, CRP-associated genes such as FAM20A, as well as the JAK-STAT pathway member CISH were downregulated following treatment with FIL.
Changes in circulating cellular composition were observed following filgotinib treatment comprising a decrease in percentage of neutrophils (weeks 1, 4), a transient decrease in monocytes (weeks 1, 4), an increase in B cells (weeks 4, 12) and a transient increase in lymphocytes (weeks 1, 4). These cellular fluctuations accounted for a portion of the changes in gene expression observed, including loss of some gene expression changes over time and upregulation of many genes at weeks 4 and 12.
Conclusion: In patients with active AS, FIL treatment meaningfully decreased inflammatory pathways and genes associated with disease activity in AS, and increased TGF-β signaling pathways as a result of changes in inflammatory gene expression and alterations in circulating cellular composition.
Reference
1. van der Heijde D, Baraliakos X, Gensler LS, et al. Lancet. 2018;392:2378–87.
To cite this abstract in AMA style:
Poddubnyy D, Liu Y, Barchuk W, Besuyen R, Galien R, Tian Y, Malkov V, Hertz A. Whole Blood Transcriptional Changes Following Treatment with Filgotinib in Patients with Ankylosing Spondylitis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/whole-blood-transcriptional-changes-following-treatment-with-filgotinib-in-patients-with-ankylosing-spondylitis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/whole-blood-transcriptional-changes-following-treatment-with-filgotinib-in-patients-with-ankylosing-spondylitis/