Session Information
Date: Tuesday, November 14, 2023
Title: (1895–1912) Measures & Measurement of Healthcare Quality Poster II
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: When assessing disease activity in patients with axial spondyloarthritis (axSpA), the Ankylosing Spondylitis Disease Activity Score with C-reactive protein (ASDAS-CRP) is recommended over ASDAS with erythrocyte sedimentation rate (ASDAS-ESR). Although ASDAS-CRP and ASDAS-ESR are not interchangeable, the same cut-offs are used to classify patients into four distinct disease activity states: inactive disease (ID), low disease activity (LDA), high disease activity (HDA) and very high disease activity (VHDA). These cut-offs were developed based on ASDAS-CRP. We aimed to (i) estimate optimal ASDAS-ESR values corresponding to the established ASDAS-CRP cut-offs (< 1.3, < 2.1 and > 3.5), and (ii) investigate the improvement of level of agreement between ASDAS-ESR and ASDAS-CRP disease activity states when applying the estimated ASDAS-ESR cut-offs.
Methods: Prospectively collected real-world data from axSpA patients starting a 1st, 2nd or 3rd tumor necrosis factor inhibitor (TNFi) from 9 countries participating in the European Spondyloarthritis (EuroSpA) Research Collaboration Network were analysed. Data were collected at baseline and three follow-up visits (6, 12 and 24 months) per TNFi treatment course. To have the best representation of the disease activity states, aggregated follow-up data were used to evaluate the ASDAS-CRP cut-offs for ID and between LDA and HDA, while baseline data were used to assess the cut-off for VHDA. We performed receiver operating characteristic analyses using the Youden index to estimate the optimal ASDAS-ESR values corresponding to ASDAS-CRP cut-offs. The level of agreement between ASDAS-ESR and ASDAS-CRP disease activity states was also assessed.
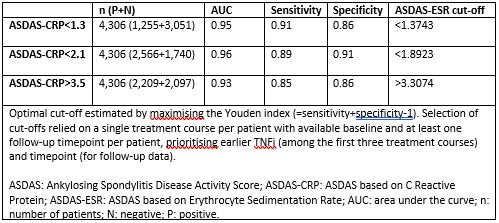
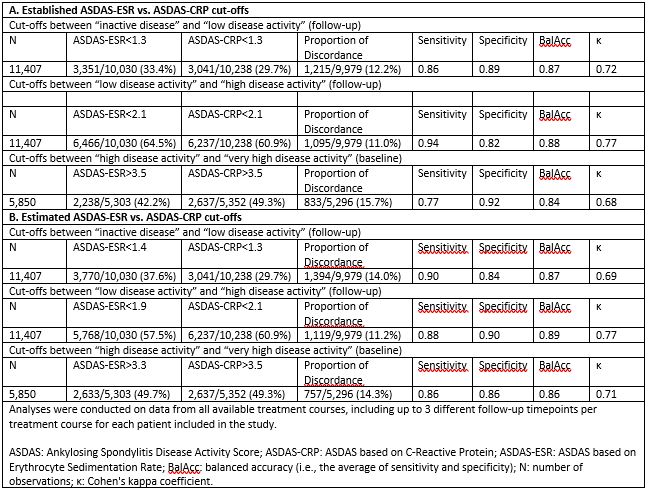
Results: We analysed data from 4,306 patients with complete registration of ASDAS scores at baseline and at least one complete follow-up registration of ASDAS scores during the same TNFi treatment course. Mean (SD) ASDAS-CRP and ASDAS-ESR were 3.5 (1.0) and 2.0 (1.0) at baseline and 3.3 (1.0) and 1.9 (1.0) at follow-up, respectively. The estimated ASDAS-ESR cut-offs between ID and LDA, between LDA and HDA and between HDA and VHDA corresponding to ASDAS-CRP cut-offs were < 1.4, < 1.9 and > 3.3, respectively (Table 1). Good agreement was observed between disease activity states applying the established ASDAS-ESR and ASDAS-CRP cut-offs, although the VHDA cut-off showed slightly worse agreement than the other two cut-offs (Table 2, upper panel). The statistical measures comparing the estimated ASDAS-ESR (< 1.4, < 1.9 and > 3.3) and ASDAS-CRP (< 1.3, < 2.1 and > 3.5) cut-off values performed slightly better only for VHDA as compared to applying the same cut-offs for both ASDAS versions (Table 2, lower panel). However, the proportion of discordance for the cut-offs for ID and between LDA and HDA increased slightly with the estimated ASDAS-ESR cut-off values.
Conclusion: In a multi-national cohort, the estimated ASDAS-ESR cut-offs between disease activity states were < 1.4, < 1.9 and > 3.3. The original cut-offs overall performed similarly as the ones we estimated. Our findings did not provide sufficient evidence to reject the established ASDAS-ESR cut-offs.
To cite this abstract in AMA style:
Georgiadis S, Ørnbjerg L, Michelsen B, Kvien T, Di Giuseppe D, Karlsson Wallman J, Zavada J, Provan S, Rodrigues A, Santos M, Rotar Z, Nordstrom D, Hokkanen A, Macfarlane G, Jones G, van der Horst-Bruinsma I, Hellamand P, Østergaard M, Hetland M. Which ASDAS-ESR Cut-offs for Disease Activity Correspond to ASDAS-CRP Cut-offs in Axial Spondyloarthritis? – Results from the EuroSpA Collaboration [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/which-asdas-esr-cut-offs-for-disease-activity-correspond-to-asdas-crp-cut-offs-in-axial-spondyloarthritis-results-from-the-eurospa-collaboration/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/which-asdas-esr-cut-offs-for-disease-activity-correspond-to-asdas-crp-cut-offs-in-axial-spondyloarthritis-results-from-the-eurospa-collaboration/