Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: The American College of Rheumatology (ACR) response definition was developed in 1995 by Felson et al. as a composite outcome and has been the most frequently used response definition in rheumatoid arthritis (RA) clinical trials. The ACR20 response is usually required as a primary outcome to be used for approval of investigative drugs by the FDA. However, in the recent past this threshold has been criticized for its lack of clinical relevance, and higher thresholds, such as ACR50 or ACR70 responses might be better suited.
In this analysis we investigated the consistency of ACR response definitions (20%, 50% or 70%) over the duration of individual trials, and the power to differentiate between active treatment and placebo.
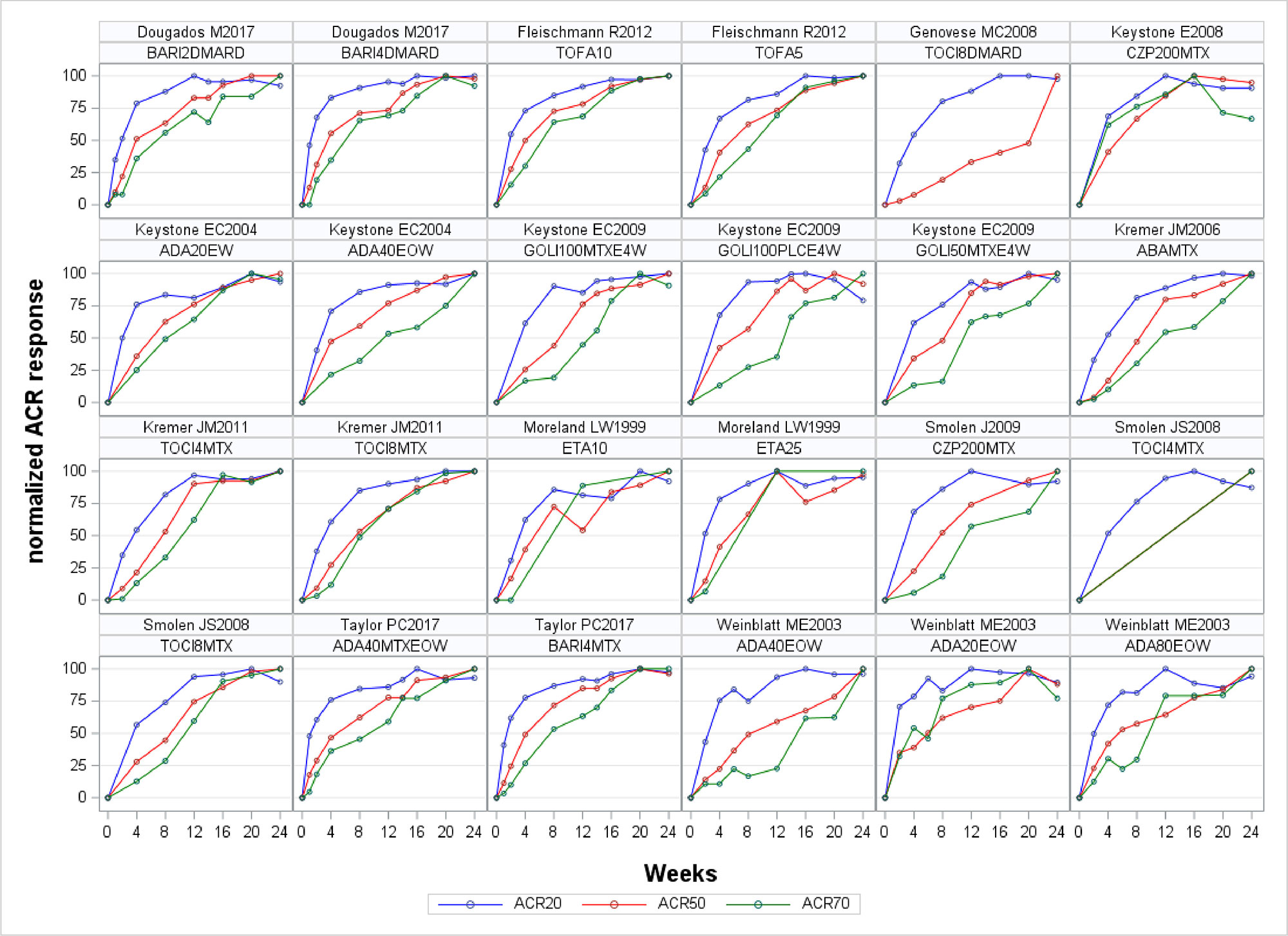
Methods: Based on a systematic literature search using (EMBASE, Medline and the Cochrane Library) we identified all published articles on multi-national, multi-center, phase 3 trials in adult RA patients who had active disease despite methotrexate therapy, investigating biological disease modifying anti-rheumatic drugs (bDMARD) and targeted synthetic (ts)DMARDs. We only included articles published in English language. All ACR outcomes available in the public domain were extracted using standardized spreadsheets. ACR 20%, 50% and 70% response rates were overlay plotted graphically on scales normalised to their maximum response to visually explore differences in timing of response. We then utilized Cochran-Mantel-Haenszel (CMH) statistics to calculate p-values per outcome and timepoint for each trial individually. Statistics and visualizations were done using SAS 9.4M5 (Cary, New York, USA).
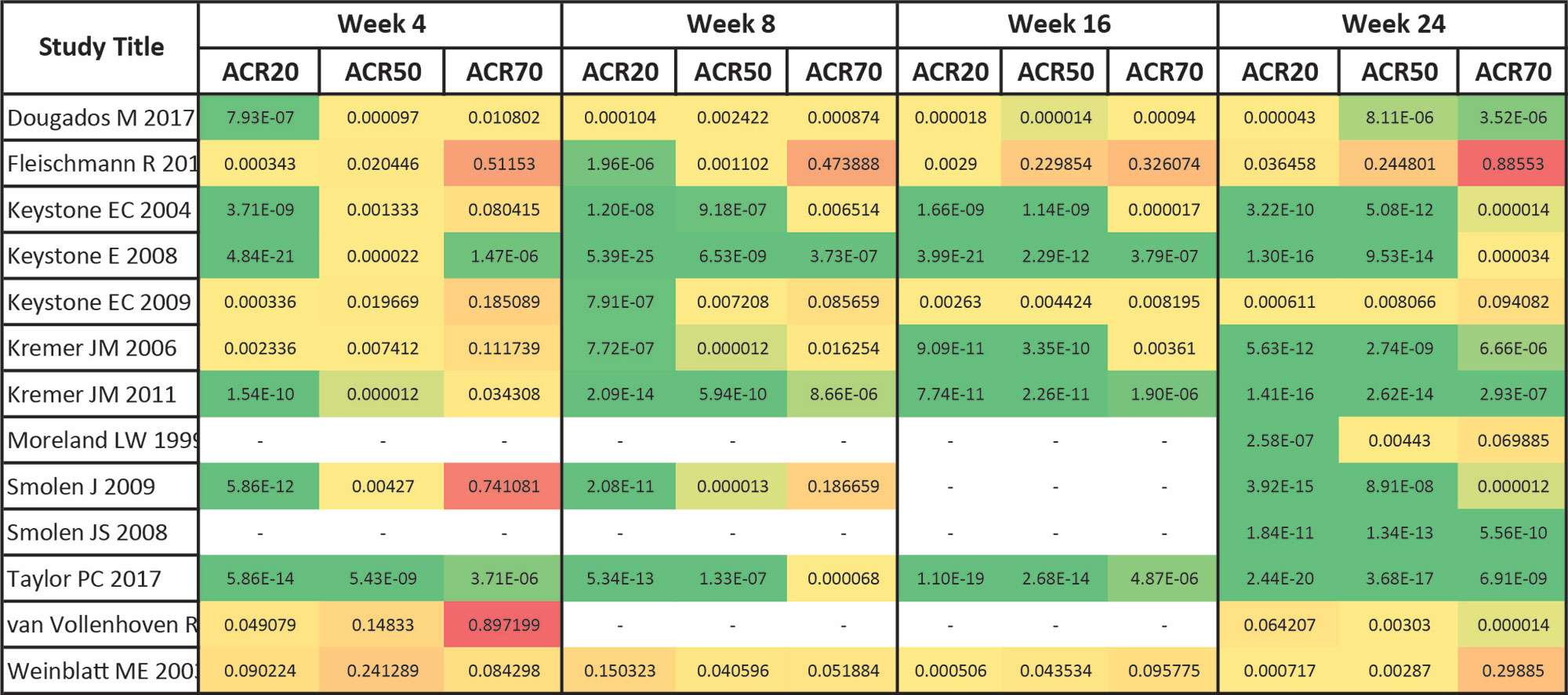
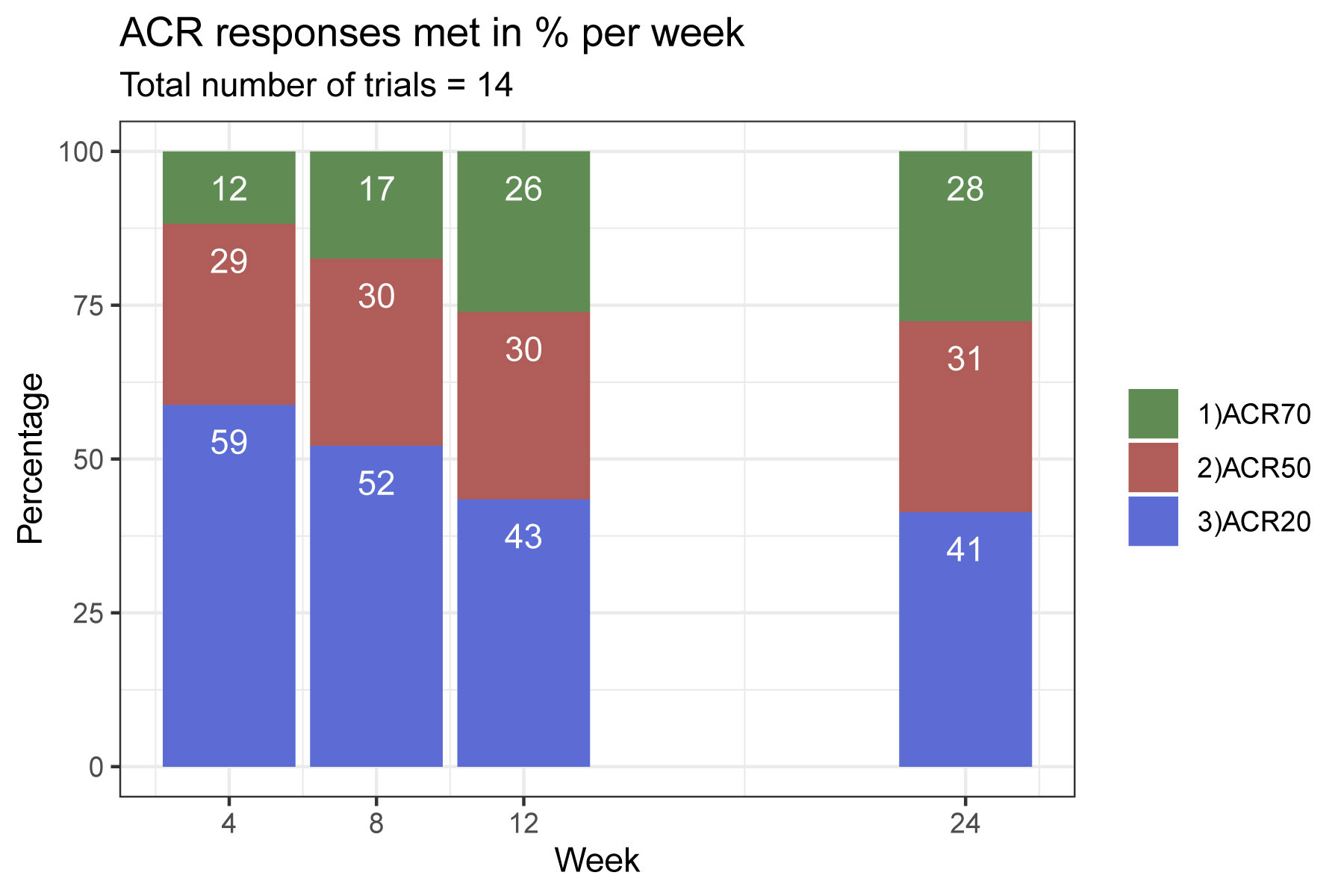
Results: 12457 articles were screened; most of the papers were excluded because of insufficient reporting of ACR outcomes (such as no time course), trials drug ultimately not being of multi-center/multi-national nature or investigational drugs that were not licensed for use in RA. We ultimately included 14 trials in the analysis, investigating 46 treatment arms of 8 different licensed compounds. ACR20 had the highest discriminant power across the trial duration, followed by ACR50, and ACR70 (Figure 1). The ACR20 also showed best discrimination at early time points and the ACR70 at the latest ones, as evident from the depiction of response rates across thresholds, which were normalized to the maximum response within each threshold (Figure 2). p-values, as shown for individual trials for each outcome, also suggest that ACR20% rates lead to more pronounced statistical effects than ACR50% or ACR70% responses over time (Figure 3). For trials achieving significant ACR20% responses, only 3/14 (21%) of trials had no significant ACR50 response at the given time point, and this number was 10/14 (71%) for ACR70.
Conclusion: Our analyses suggest that the ACR20 response rate is superior to the ACR 50 and ACR 70 response to differentiate between active treatment and placebo in randomized controlled trials in RA, especially if primary endpoints are to be assessed at early time points, such as 12 weeks; using the clinically more relevant ACR50 and ACR70 for discrimination at later timepoints, when their response rates peak, also allowed good discrimination in the majority of studies.
To cite this abstract in AMA style:
Kerschbaumer A, Konzett V, Smolen J, Aletaha D. Which ACR Response Definition Should Be Used for Disease Activity Claims in Approval Studies of Rheumatoid Arthritis? A Systematic Evaluation [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/which-acr-response-definition-should-be-used-for-disease-activity-claims-in-approval-studies-of-rheumatoid-arthritis-a-systematic-evaluation/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/which-acr-response-definition-should-be-used-for-disease-activity-claims-in-approval-studies-of-rheumatoid-arthritis-a-systematic-evaluation/