Session Information
Session Type: Poster Session B
Session Time: 5:00PM-6:00PM
Background/Purpose: Tumor necrosis factor alpha (TNF-alpha) induced protein 3 gene, or TNFAIP3, encodes the A20 protein, an important regulator of the NF-κB pathway. Since its initial report in 2016, A20 haploinsufficiency has been described as a human autoinflammatory disease caused by heterozygous loss-of-function mutations in TNFAIP3 leading to insufficient suppression of NF-kB activity. Previous studies suggested that mutated A20 was degraded, and the phenotype was caused by insufficient NF-kB canonical pathway modulation. This study aims to determine the effect of the L227X mutation in A20 protein expression, using different cell lines.
Methods: Green fluorescent protein (GFP)-tagged plasmids were constructed with the TNFAIP3 gene and a L227X mutation was induced using QuikChange II XL Site-Directed Mutagenesis Kit (Agilent®, Santa Clara, USA). Mutated plasmids were transfected into THP-1 (human monocytic), Jurkat (human lymphoblastic T cells) and HL-60 (human neutrophilic) cells, using a viral vector. THP-1 cells were also transfected with an empty plasmid (EP, only the backbone and GFP) or GFP-tagged wild-type TNFAIP3 plasmid (TNFAIP3-WT). Original genetic content was not altered (no knock-out was performed). Cells were assessed for GFP expression, after multiple passages, to ensure stable plasmid incorporation. A20 was stained by a C-terminal monoclonal antibody against TNFAIP3 (Abcam®, Cambridge, UK) and protein expression assessed by flow cytometry.
Results: A20 expression was systematically reduced in mutated TNFAIP3-L227X plasmid transfected cell lines compared to non-transfected control cells: HL-60 (MFI=572 vs 450, p 0.05, figure 1) and Jurkat (MFI=764 vs 695, p 0.01, figure 2). A20 protein expression was significantly reduced in TNFAIP3-L227X transfected THP-1 cells (MFI=581) compared to non-transfected (MFI=1168), EP-transfected (MFI=1321) or TNFAIP3-WT transfected cells (MFI=1395, p 0.01; figure 3). TNFAIP3-WT showed a modest but significant increase of A20 expression compared to non-transfected THP-1.
Conclusion: Stable TNFAIP3-L227X transfection in human cell lines was able to induce reduction of A20 protein expression, in the absence of gene silencing or knock-out of endogenous TNFAIP3. This suggests TNFAIP3-L227X mutation might act through other mechanisms beyond haploinsufficiency in immune cell lines, which merits further investigation.
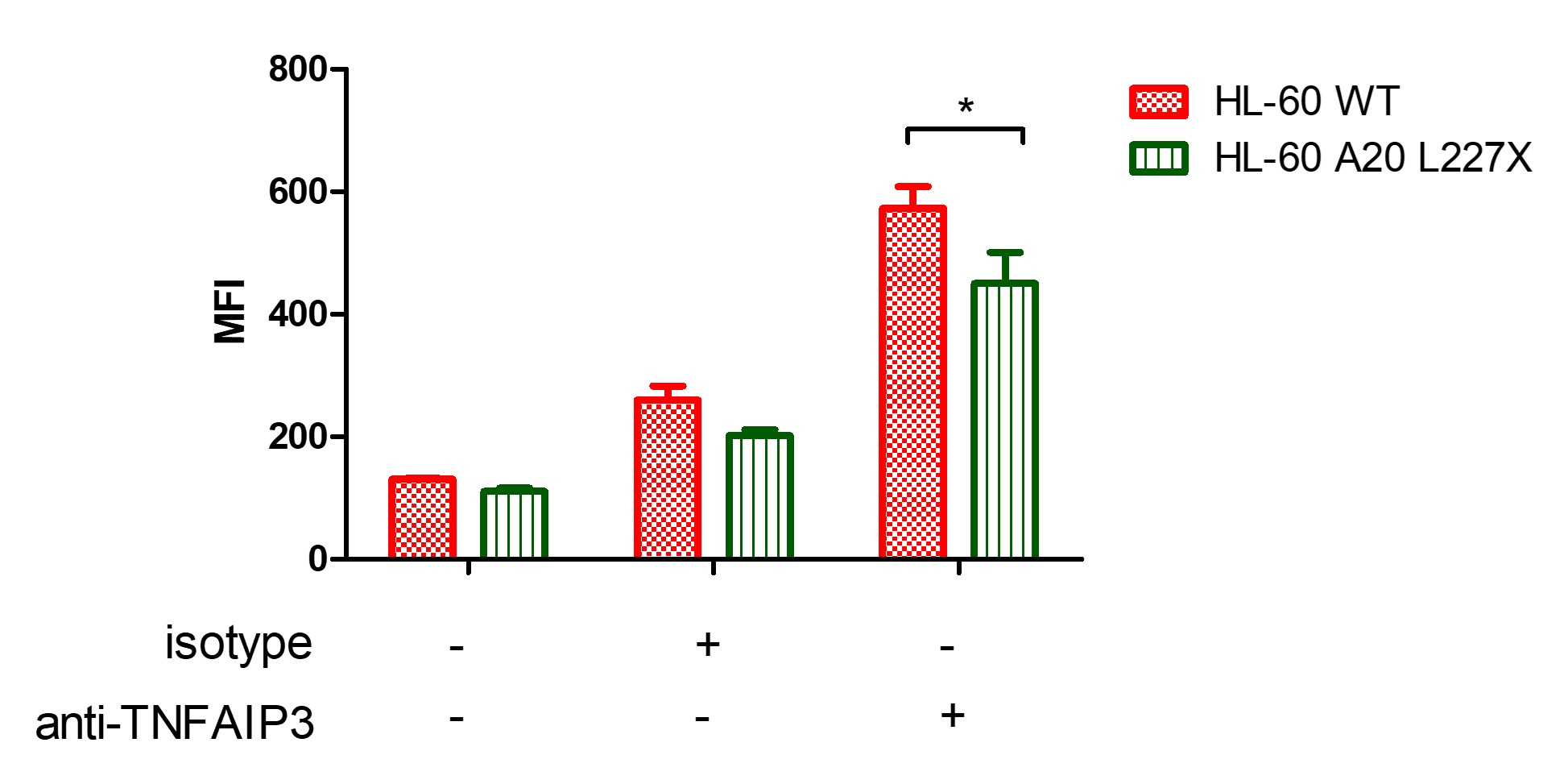 Figure 1: A20 protein expression in HL-60 cells, non-transfected (red) or transfected with TNFAIP3-L227X plasmid (green). * p < 0.05
Figure 1: A20 protein expression in HL-60 cells, non-transfected (red) or transfected with TNFAIP3-L227X plasmid (green). * p < 0.05
 Figure 2: A20 protein expression in Jurkat cells, non-transfected (red) or transfected with TNFAIP3-L227X plasmid (green). ** p < 0.01
Figure 2: A20 protein expression in Jurkat cells, non-transfected (red) or transfected with TNFAIP3-L227X plasmid (green). ** p < 0.01
 Figure 3: A20 protein expression in THP_1 cells, non-transfected (red), transfected with empty plasmid (grey), transfected with wild-type TNFAIP3 plasmid (blue) or transfected with TNFAIP3-L227X plasmid (green). ** p < 0.01; ***p < 0.001.
Figure 3: A20 protein expression in THP_1 cells, non-transfected (red), transfected with empty plasmid (grey), transfected with wild-type TNFAIP3 plasmid (blue) or transfected with TNFAIP3-L227X plasmid (green). ** p < 0.01; ***p < 0.001.
To cite this abstract in AMA style:
Pontes Aires P, PIOTTO D, Cunha A, Perazzio S, Terreri M. What’s in a Name? A20 Protein Expression in an in Vitro Model of A20 Haploinsufficiency [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 4). https://acrabstracts.org/abstract/whats-in-a-name-a20-protein-expression-in-an-in-vitro-model-of-a20-haploinsufficiency/. Accessed .« Back to 2023 Pediatric Rheumatology Symposium
ACR Meeting Abstracts - https://acrabstracts.org/abstract/whats-in-a-name-a20-protein-expression-in-an-in-vitro-model-of-a20-haploinsufficiency/
