Session Information
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: Multiple RA therapies are available that differ in attributes such as mode of administration and benefit-risk profile. Challenging trade-offs are made during treatment selection to accommodate patients’ circumstances and ensure comprehensive disease management. EULAR recommendations for RA management emphasize the need to recognize patient preferences in shared decision-making (SDM). This study elicited trade-offs that RA patients were willing to make during treatment selection, accounting for preference heterogeneity.
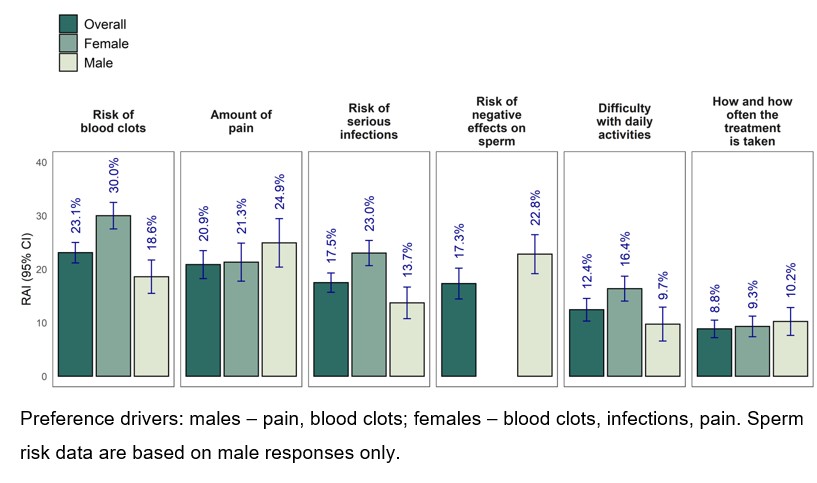
Methods: An online discrete choice experiment was conducted from Sep–Oct 2021; RA patients were required to elicit their preferences for RA treatment attributes (Figure) and make trade-offs between them. Attributes were chosen based on literature review and qualitative patient interviews; these were tested in a quantitative pilot. Main data collection was via an online survey which asked participants to choose between hypothetical treatments. Patients were ≥18 years old, diagnosed with RA, currently received systemic DMARD therapy for RA, and resident in France, Germany, Italy, Spain, United Kingdom, or United States. Male patients were oversampled to support subgroup analysis of preferences for effects on sperm parameters. Data were analyzed using a correlated mixed logit model; differences in preferences by sex and age were explored. Relative attribute importance (RAI) scores and maximum acceptable risk (MAR) measures were derived.
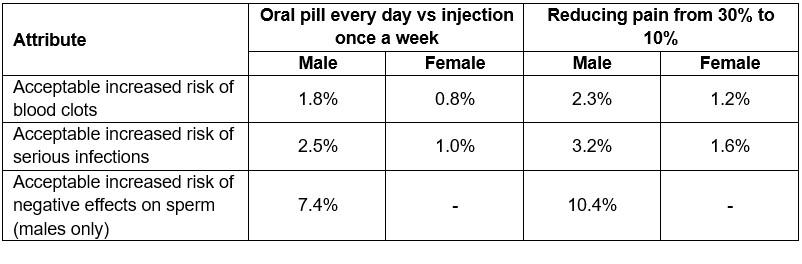
Results: In total, 2,090 patients participated; 42% were female with predefined oversampling of males; mean age was 45.2 years (range 18–83). Estimated effects were significant for all attributes (p< 0.001), implying that they all influenced treatment choice and that preferences differed between participants. RAI scores revealed different priorities between males and females (Figure). Reducing pain and negative effect on semen parameters was most important to males; females were most concerned by risk of blood clots and serious infections. Remaining attributes were of lower importance. No single attribute explained treatment preferences by more than 30%. Patients aged 18-44 years placed less importance on frequency and mode of treatment administration than older patients. Patients accepted extra risks of blood clots, serious infections, or negative effects on sperm for an oral pill every day vs injection once a week, and for reducing amount of pain from 30% to 10% (Table). Similar observations were made for improved performance of daily activities. Acceptable trade-offs varied between patients.
Conclusion: Preferences of RA patients were driven by benefits and risks of RA treatments, with no single attribute dominating the decision making. Patients were willing to accept higher risk of serious infections and blood clots in exchange for improvements in pain, daily activities, or administration convenience. These findings emphasize the importance of considering the entire treatment profile, including benefits, risks, and administration to support SDM between providers and patients.
To cite this abstract in AMA style:
Alten R, Nieto-Gonzalez J, Jacques P, Montecucco C, Moots R, Radner H, Heidenreich S, Whichello C, Krucien N, Zignani M, Vonkeman H, Van Beneden K. What Trade-offs Are Acceptable to Rheumatoid Arthritis Patients During Treatment Selection? [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/what-trade-offs-are-acceptable-to-rheumatoid-arthritis-patients-during-treatment-selection/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/what-trade-offs-are-acceptable-to-rheumatoid-arthritis-patients-during-treatment-selection/