Session Information
Date: Monday, November 8, 2021
Title: Patient Outcomes, Preferences, & Attitudes Poster III: Patient Preferences (1153–1169)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: The market share of biosimilars (bsDMARDs) is steadily growing, not only in rheumatologic care. Although data on efficacy, efficiency and safety have been generated in the last years, not much is known about patients’ views on bsDMARDs and also the experience with multiple switching in a non-medical switch scenario is limited. The aim of this project was to study differences in patient satisfaction after education by rheumatologists or nurse specialists, and to learn more about patients’ knowledge and their view on biosimilars.
Methods: The non-medical switch to adalimumab MSB 11022 was taken as an opportunity to study patients’ knowledge and views on bsDMARDs and their satisfaction with the information they had received on this subject. Adult patients of a tertiary hospital with chronic inflammatory rheumatic diseases (CIRD) such as rheumatoid arthritis (RA), axial spondyloarthritis (axSpA) and psoriatic arthritis (PsA) were included in this randomised controlled trial after obtaining informed consent and followed up for 3 months. Patients with other serious diseases and language problems were excluded. Patients were randomized into two groups using block randomization: Group 1: educational process by nurse specialist (nurse led clinic (NLC)), Group 2: educational process by rheumatologist (rheumatologist led clinic (RLC)). Standard assessments using validated outcome parameters for disease activity and physical function were used. Patient’s satisfaction with care was assessed by the Leeds Satisfaction Questionnaire (LSQ) which contains items on 5 subscales: provision of information, empathy with and attitude to the patient, access to and continuity with the care giver, technical competence and a general satisfaction scale. A structured questionnaire was used to assess patient’s knowledge of the manufacturing, effectiveness and safety, acceptance and cost of bsDMARDS.
Results: A total of 102 patients were included, 40 (39.2%) by the rheumatologist and 62 (60.8%) were seen by the nurse specialist. Fifty patients (49%) had undergone one and 52 multiple switches (51%). Patient demographics, disease characteristics and the results including patients’ knowledge about bsDMARDs and satisfaction with the information process are shown in Table 1. Scores of disease activity and physical function obtained at week 12 remained unchanged for 3 months. Less than one third of patients was able to correctly answer questions about manufacturing, efficacy and safety, approval and costs of bsDMARDs (Fig.1). Patients were generally satisfied with care irrespective of whether information about bsDMARDs and switching had been given by the nurse or the rheumatologist.
Conclusion: This study shows that the satisfaction and the outcomes of patients informed on bsDMARDs by a nurse were not different from information provided by the rheumatologist. Multiswitching did not lead to reduced satisfaction with care in patients on bsDMARDs, and the number of switches did not have a negative impact on patients’ satisfaction. However, patients’ knowledge on bsDMARDs was limited.
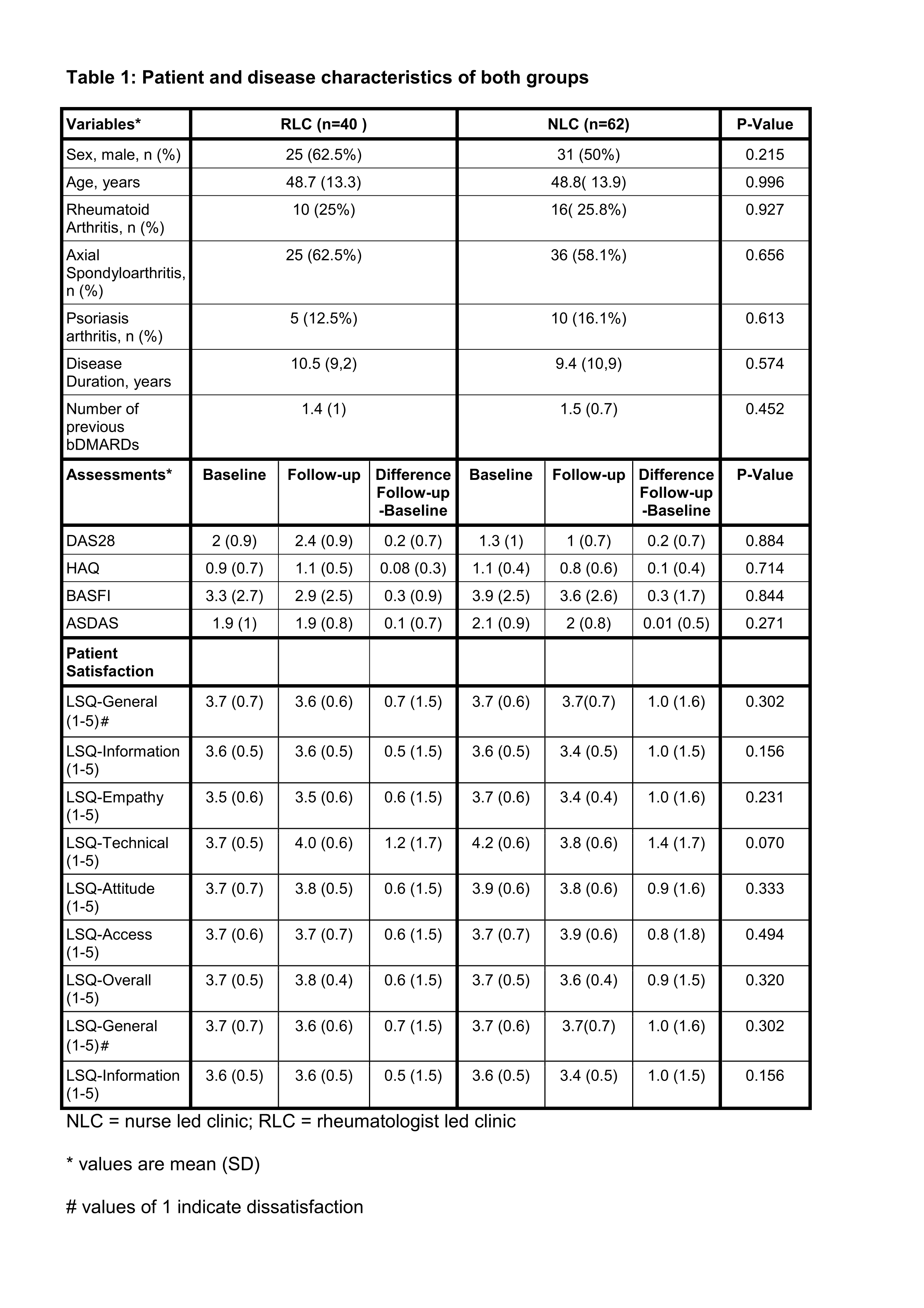 Table title on top: Table 1: Patient and disease characteristics of both groups Table legend on the bottom: NLC = nurse led clinic; RLC = rheumatologist led clinic * values are mean (SD) # values of 1 indicate dissatisfaction
Table title on top: Table 1: Patient and disease characteristics of both groups Table legend on the bottom: NLC = nurse led clinic; RLC = rheumatologist led clinic * values are mean (SD) # values of 1 indicate dissatisfaction
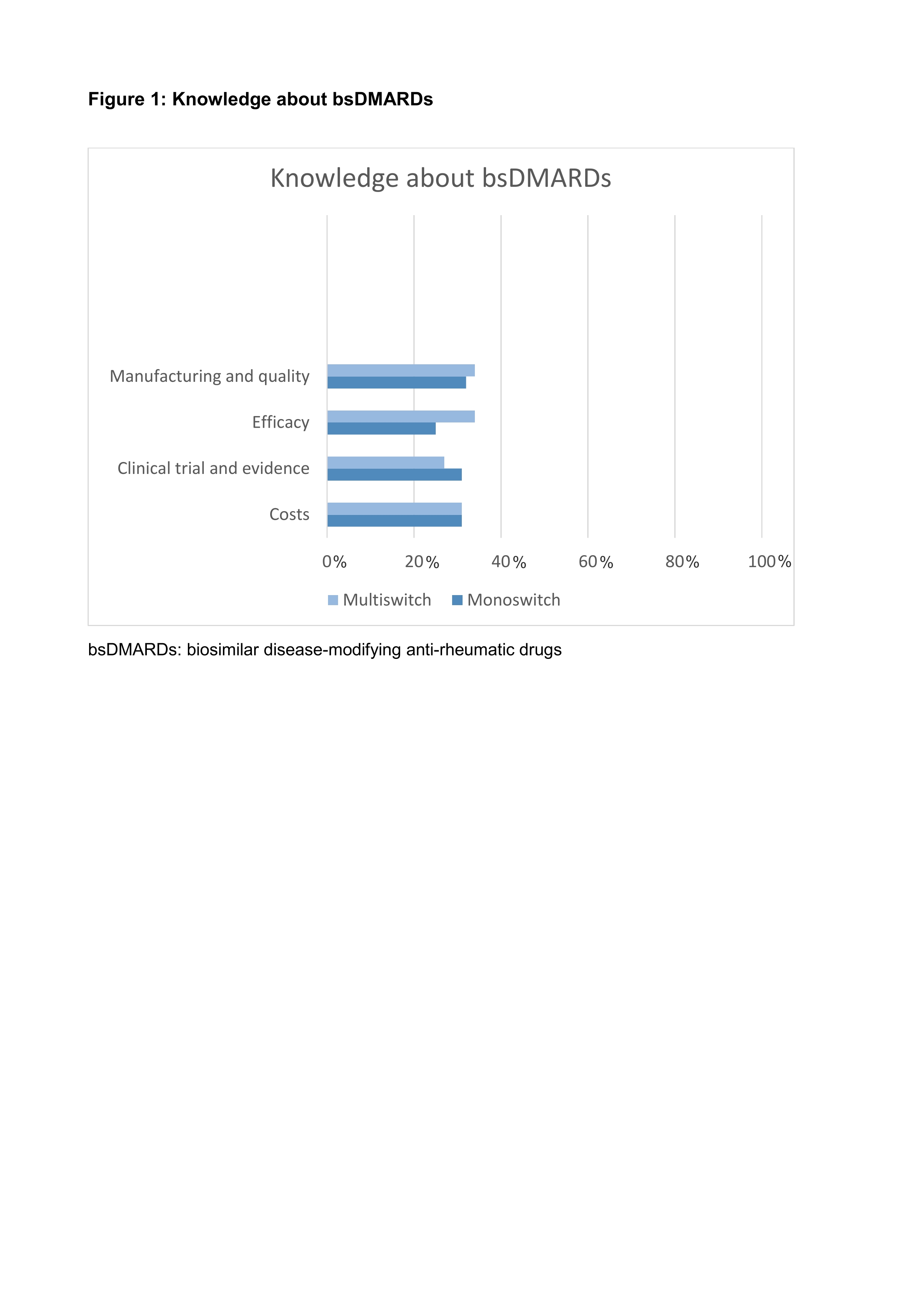 Figure title on top: Figure 1: Knowledge about bsDMARDs Figure legend on the bottom: bsDMARDs: biosimilar disease-modifying anti-rheumatic drugs
Figure title on top: Figure 1: Knowledge about bsDMARDs Figure legend on the bottom: bsDMARDs: biosimilar disease-modifying anti-rheumatic drugs
To cite this abstract in AMA style:
Gall S, Kiltz U, Kobylinski T, Andreica I, Vaupel K, Baraliakos X, Braun J. What Do Patients Know About Biosimilars and How Satisfied Are They with the Educational Process? – A Systematic Comparison Between Rheumatologists and Nurse Specialists, Including Effects of Multiswitching [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/what-do-patients-know-about-biosimilars-and-how-satisfied-are-they-with-the-educational-process-a-systematic-comparison-between-rheumatologists-and-nurse-specialists-including-effects-of-multiswit/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/what-do-patients-know-about-biosimilars-and-how-satisfied-are-they-with-the-educational-process-a-systematic-comparison-between-rheumatologists-and-nurse-specialists-including-effects-of-multiswit/
