Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The Routine Assessment of Patient Index Data 3 (RAPID3) score is a composite of 3 patient-reported outcomes (PROs): Health Assessment Questionnaire (HAQ), pain visual analog scale, and patient global assessment. Due to its simple scoring, RAPID3 is easy to use, useful for remotely monitoring patients (pts), and provides a quick, quantitative assessment of pt-perceived disease burden in psoriatic arthritis (PsA). However, data reporting the utility of RAPID3 in pts with oligoarticular (oligo) PsA is sparse. This post hoc analysis used RAPID3 to assess disease severity of early oligo PsA in the FOREMOST trial.
Methods: FOREMOST (NCT03747939), a phase 4, multicenter, randomized, double-blind, placebo (PBO)-controlled trial, enrolled 308 pts with early (duration ≤5 years) oligo ( >1 but ≤4 tender joint count and swollen joint count) PsA (per Classification Criteria for Psoriatic Arthritis). Pts were randomized 2:1 to apremilast 30 mg BID (APR; n=203) or PBO (n=105) for 24 weeks (early escape at Week [W] 16), followed by an extension phase in which all pts received APR through W48. We calculated RAPID3 scores (range, 0–30) using PROs collected during the study (including the HAQ Disability Index) and report mean reduction over time, proportion of pts with a baseline score ≥3.8 achieving the minimal clinically important difference (MCID; ≥3.8 decrease from baseline), and proportion of pts with a baseline score >6 achieving low disease severity (score >3 to ≤6) or near-remission (score ≤3), with comparisons to other measures of disease activity at W16 (all randomized pts) and W48 (pts entering the extension phase).
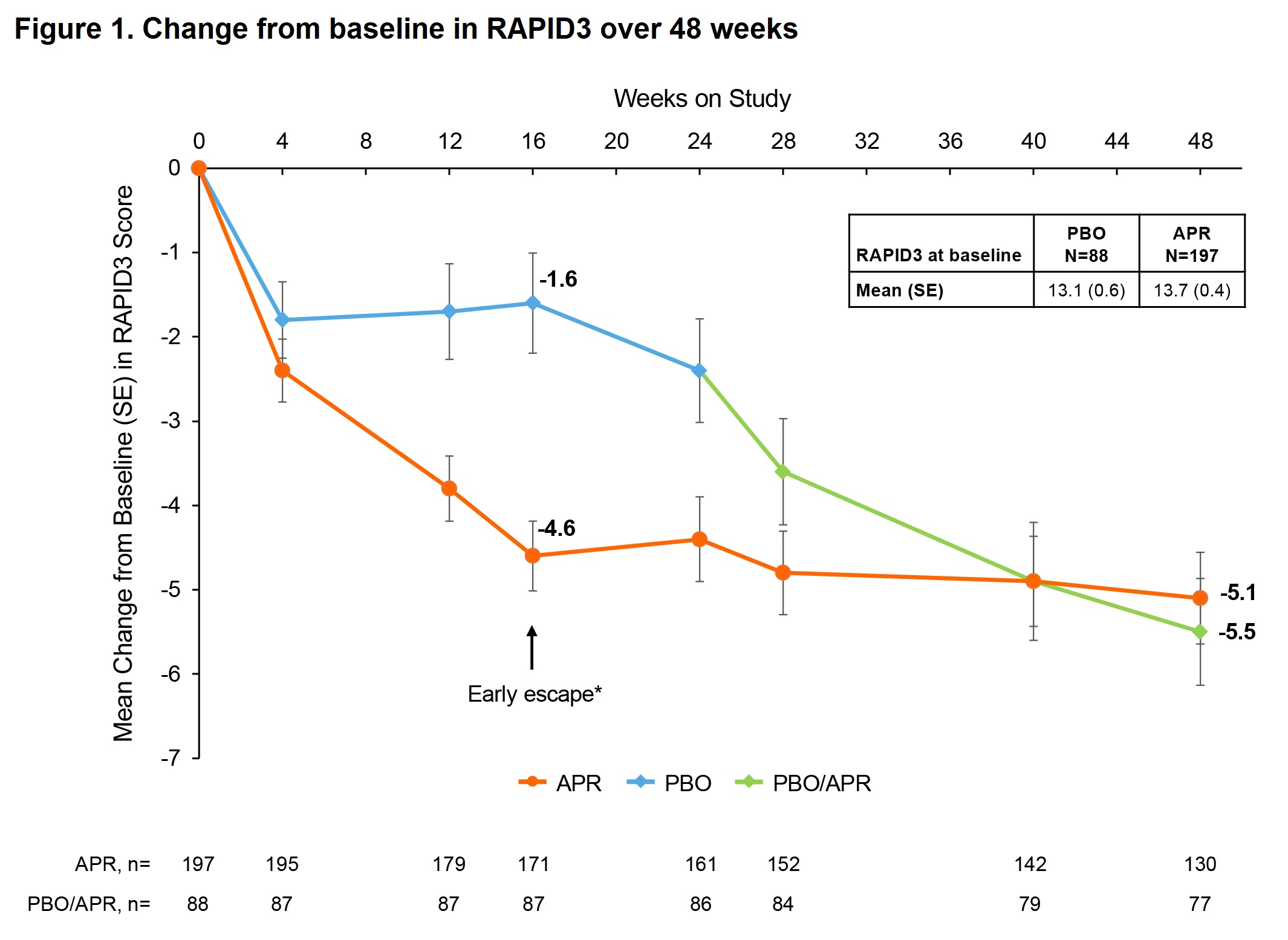
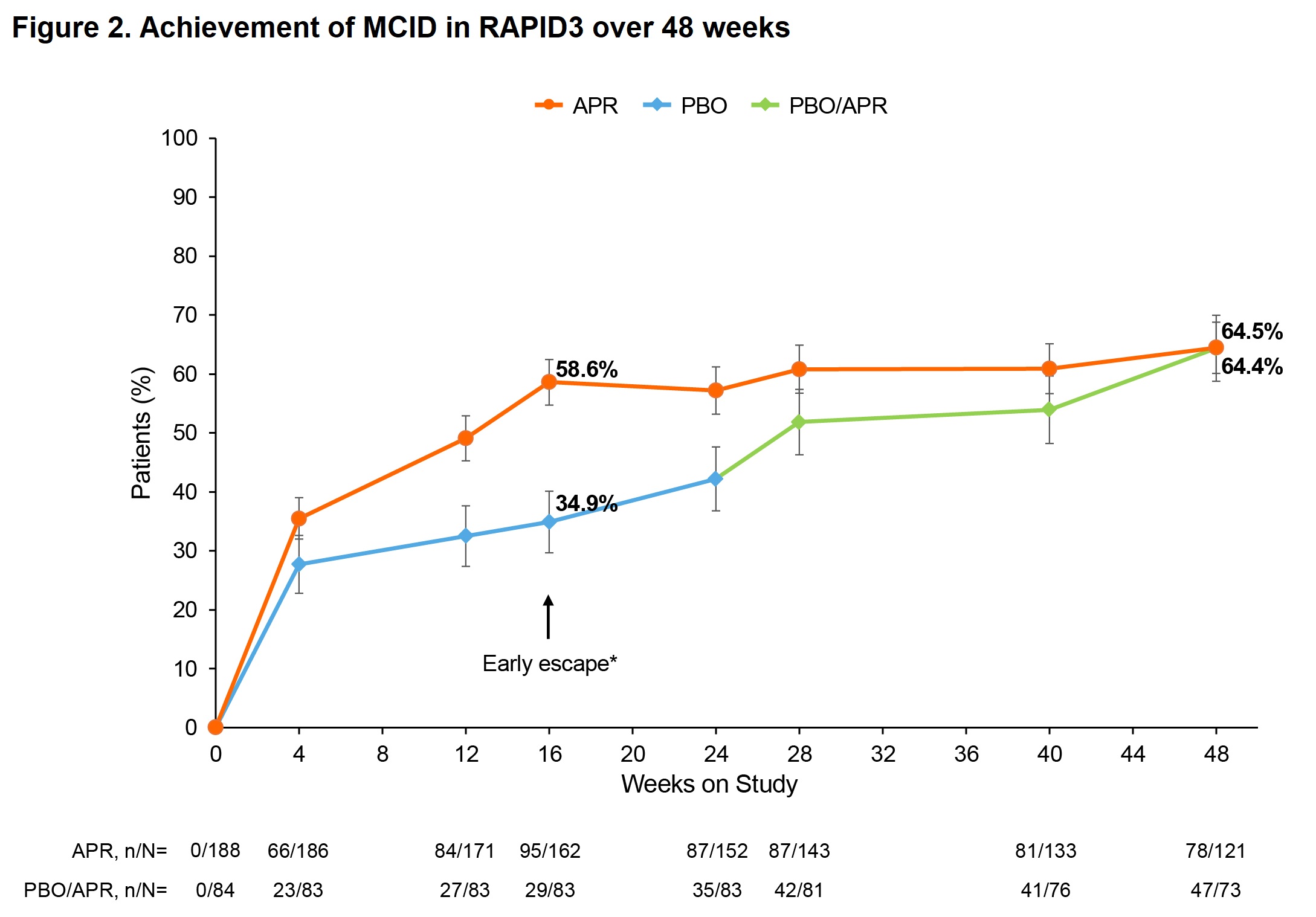
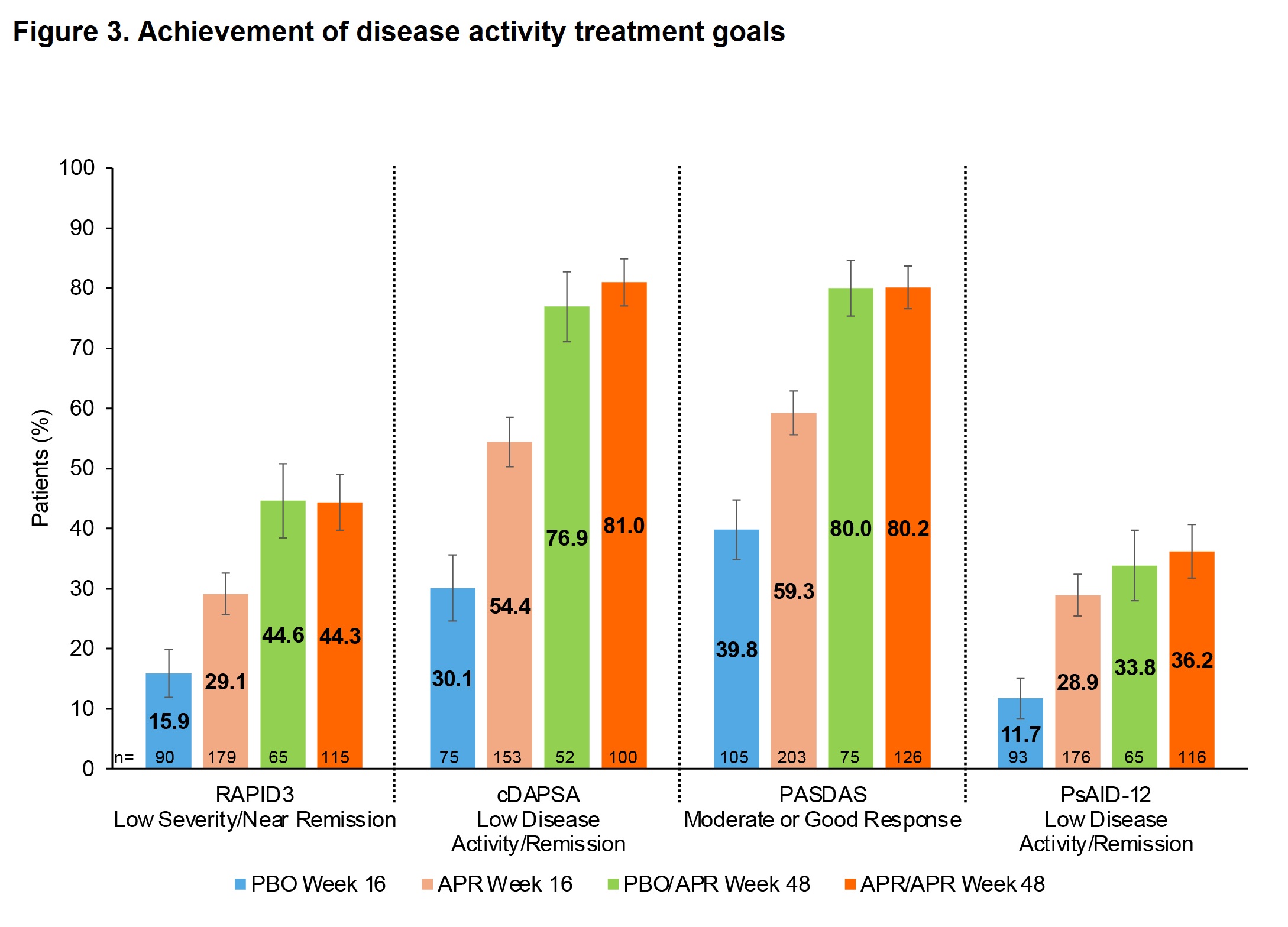
Results: Mean (standard error [SE]) baseline RAPID3 score was similar for APR (n=197) and PBO (n=105): 13.6 (0.5) vs 13.7 (0.4). At W16, the least-squares (LS) mean (95% confidence interval [CI]) change from baseline in RAPID3 score was −1.4 (−2.7, −0.1) for PBO vs −4.3 (−5.3, −3.2) for APR; treatment difference, −2.9 (95% CI: −4.2, −1.6; P< 0.0001). More pts in the APR group achieved the MCID of ≥3.8 at W16 vs PBO: 53.3% vs 31.3%; treatment difference, 22.3% (95% CI: 10.6, 34.0; P=0.0005). Similarly, more pts in the APR group achieved RAPID3 near-remission/low severity at W16 vs PBO: 29.1% vs 15.9%; treatment difference, 13.6% (95% CI: 3.2, 24.1; P=0.0171). RAPID3 scores continued to improve in pts receiving APR through W48 (Figure 1). Achievement of MCID and near-remission/low severity further improved, with approximately two-thirds of pts achieving MCID at W48 (Figure 2) and 44–45% achieving near-remission/low severity (Figure 3). Similar trends were seen with other measures of disease activity (Figure 3).
Conclusion: RAPID3 is the most commonly used PRO in US rheumatology practice and allows for simple and clinically useful measurement of disease burden. Our data from FOREMOST demonstrate a clinically relevant difference between PBO and APR in pts with early oligo PsA. Despite low joint involvement, RAPID3 scores indicate these pts had high baseline disease burden. Treatment with APR for up to 48 weeks resulted in >60% of pts achieving the MCID and near-remission/low severity in ~45%. RAPID3 response trends aligned with those observed with other clinical assessments.
RAPID3, Routine Assessment of Patient Index Data 3; range 0–30; higher scores indicate more disease activity; ≤3, near-remission; >3 to ≤6, low severity; >6 to ≤12, moderate severity; >12 to 30, high severity.
*Patients who underwent early escape at Week 16 (PBO, n=24; APR, n=21) were analyzed at Week 24 per randomization.
APR, apremilast; PBO, placebo; RAPID3, Routine Assessment of Patient Index Data 3; SE, standard error.
RAPID3, Routine Assessment of Patient Index Data 3; range 0–30; higher scores indicate more disease activity; ≤3, near-remission; >3 to ≤6, low severity; >6 to ≤12, moderate severity; >12 to 30, high severity.
*Patients who underwent early escape at Week 16 (PBO, n=23; APR, n=19) were analyzed at Week 24 per randomization.
APR, apremilast; MCID, minimal clinically important difference; PBO, placebo; SE, standard error.
W16: Patients in the full analysis set with baseline RAPID3 score >6, cDAPSA >13, or PsAID_12 >1.95, where applicable; multiple imputation or nonresponder imputation used; assessed based on all joints for cDAPSA and PASDAS.
W48: Data as observed for patients with baseline RAPID3 score >6, cDAPSA >13, or PsAID_12 >1.95, where applicable, and entering the extension; analyzed by original randomization; assessed based on sentinel joints (those affected at baseline) for cDAPSA and PASDAS.
cDAPSA, Clinical Disease Activity in Psoriatic Arthritis; range 0–154; higher scores indicate more disease activity, scores ≤13 indicate low disease activity/remission.
PASDAS, Psoriatic Arthritis Disease Activity Score; range 0–10; higher scores indicate worse disease activity; good response defined as score ≤3.2 with ≥1.6 improvement from baseline; moderate response defined as score >3.2 with ≥1.6 improvement from baseline, or score <5.4 with improvement ≥0.8 and <1.6.
PsAID_12, Psoriatic Arthritis Impact of Disease 12-item; range 0 (best status) to 10 (worst status); scores ≤1.95 indicate low disease activity/remission.
RAPID3, Routine Assessment of Patient Index Data 3; range 0–30; higher scores indicate more disease activity; ≤3, near-remission; >3 to ≤6, low severity; >6 to ≤12, moderate severity; >12 to 30, high severity.
APR, apremilast; CI, confidence interval; PBO, placebo; SE, standard error.
To cite this abstract in AMA style:
Wells A, Kavanaugh A, Tillett W, Helliwell P, Vis M, Klyachkin Y, Deignan C, Teng L, Wang R, Ogdie A. Value of the Routine Assessment of Patient Index Data 3 in Assessing Disease Severity and Treatment Effect in Patients with Early Oligoarticular Psoriatic Arthritis Treated with Apremilast [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/value-of-the-routine-assessment-of-patient-index-data-3-in-assessing-disease-severity-and-treatment-effect-in-patients-with-early-oligoarticular-psoriatic-arthritis-treated-with-apremilast/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/value-of-the-routine-assessment-of-patient-index-data-3-in-assessing-disease-severity-and-treatment-effect-in-patients-with-early-oligoarticular-psoriatic-arthritis-treated-with-apremilast/