Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The juvenile spondyloarthritis disease activity index (JSpADA) is a disease activity assessment tool developed for children with juvenile psoriatic arthritis (JPsA) and enthesitis related arthritis (ERA). This post-hoc analysis investigated the validity of JSpADA for children with ERA and JPsA in a prospective clinical trial setting using data from the JUNIPERA study.
Methods: JUNIPERA (NCT03031782) is a phase 3, placebo-controlled, event-driven withdrawal study investigating the safety and efficacy of secukinumab in pediatric patients with ERA and JPsA. Patients aged 2 to < 18 years who met the International League of Associations for Rheumatology criteria for ERA or JPsA for ≥6 months with active disease (≥3 active joints and ≥1 site of enthesitis) despite treatment with ≥1 non-steroidal anti-inflammatory drug or disease-modifying anti-rheumatic drug were enrolled. Patients received open label secukinumab (75 mg in patients < 50 kg, 150 mg in patients ≥50 kg) weekly for 5 weeks (including Week 0) followed by q4w to Week 12. At Week 12, patients were randomized 1:1 to secukinumab or placebo q4w until disease flare or Week 52. JSpADA consists of 8 equally weighted components (range, 0-8): active joint count, enthesitis count, patient-reported pain, inflammatory markers, morning stiffness, clinical sacroiliitis, uveitis, and back mobility. For this post hoc analysis, the validity of JSpADA was assessed using 3 criteria: discriminatory validity based on JSpADA scores at Week 12 among patients with active or inactive disease (defined as no joints with active arthritis, CRP level within normal limits, PGA ≤10, no patient-reported morning stiffness, and no uveitis); convergent validity based on Spearman’s correlation between JSpADA and 10-joint Juvenile Arthritis Disease Activity Score (JADAS-10), clinical JADAS-10 (cJADAS-10), and physician global assessment of disease activity (PGA) at Week 12; and responsiveness to change in clinical disease activity based on change in JSpADA scores from Week 12 to Week 52 among patients who had improvement (change in PGA < 0) or worsening (change in PGA >0) of disease activity. Reported P values are nominal, and no multiplicity adjustments were made.
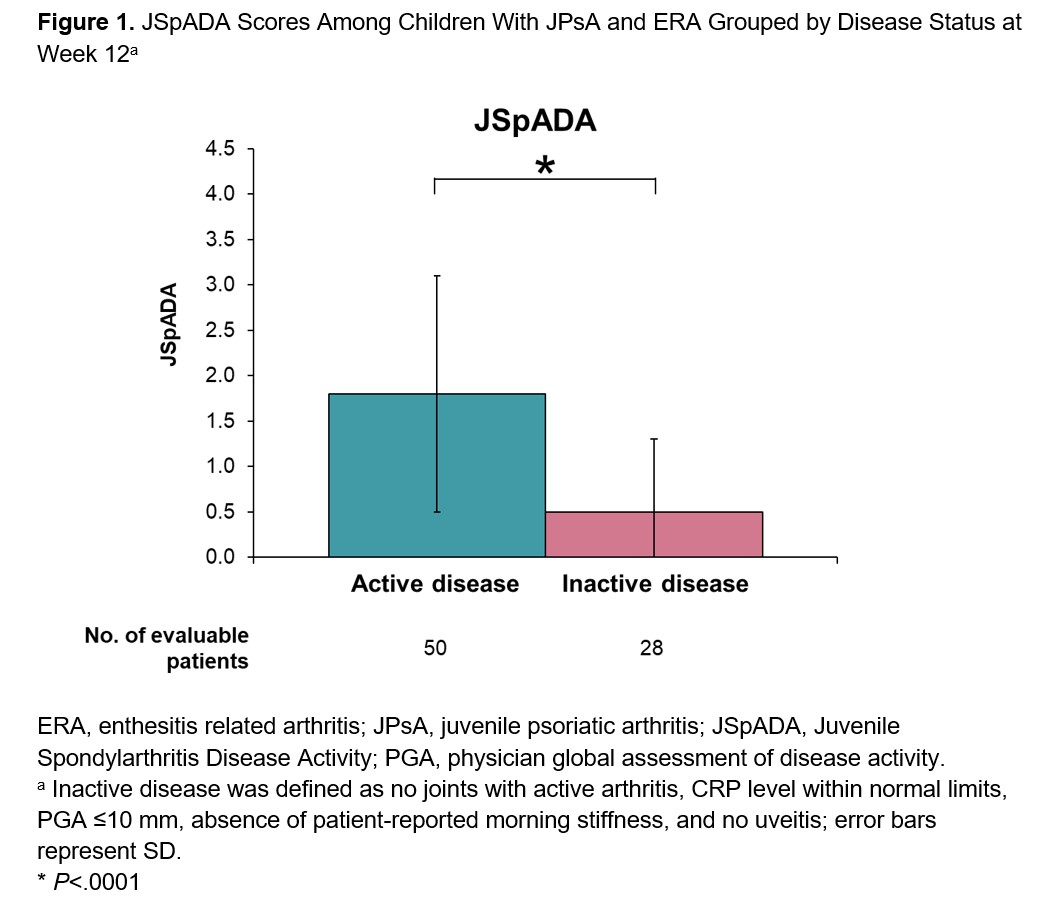
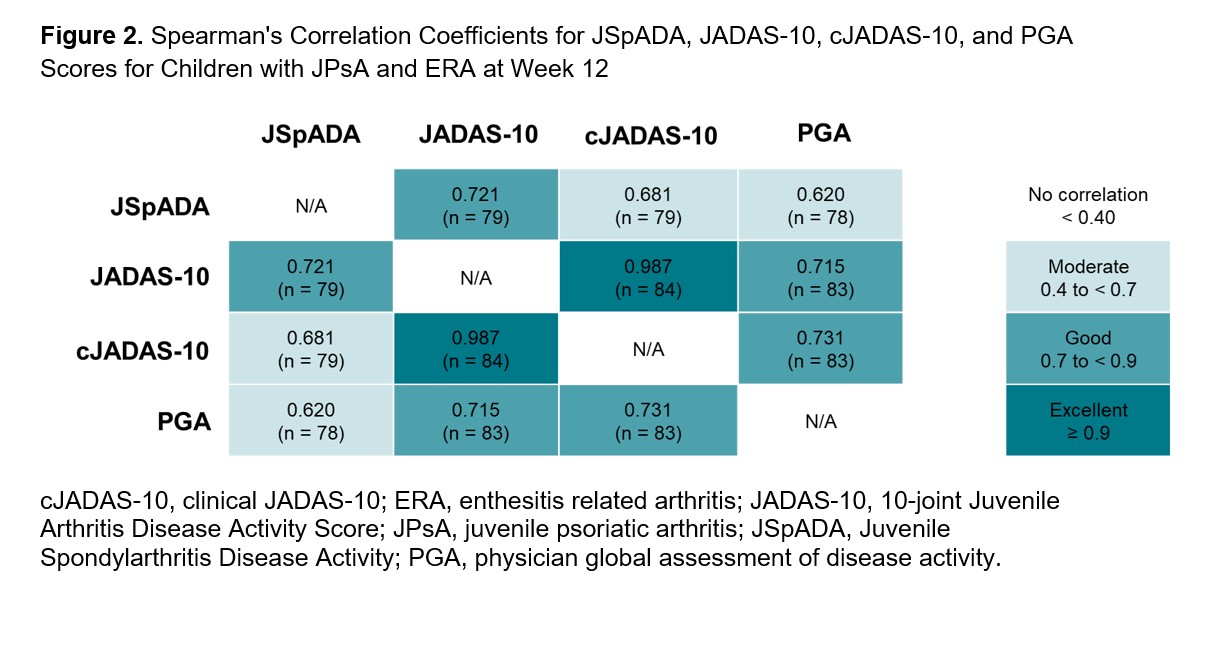
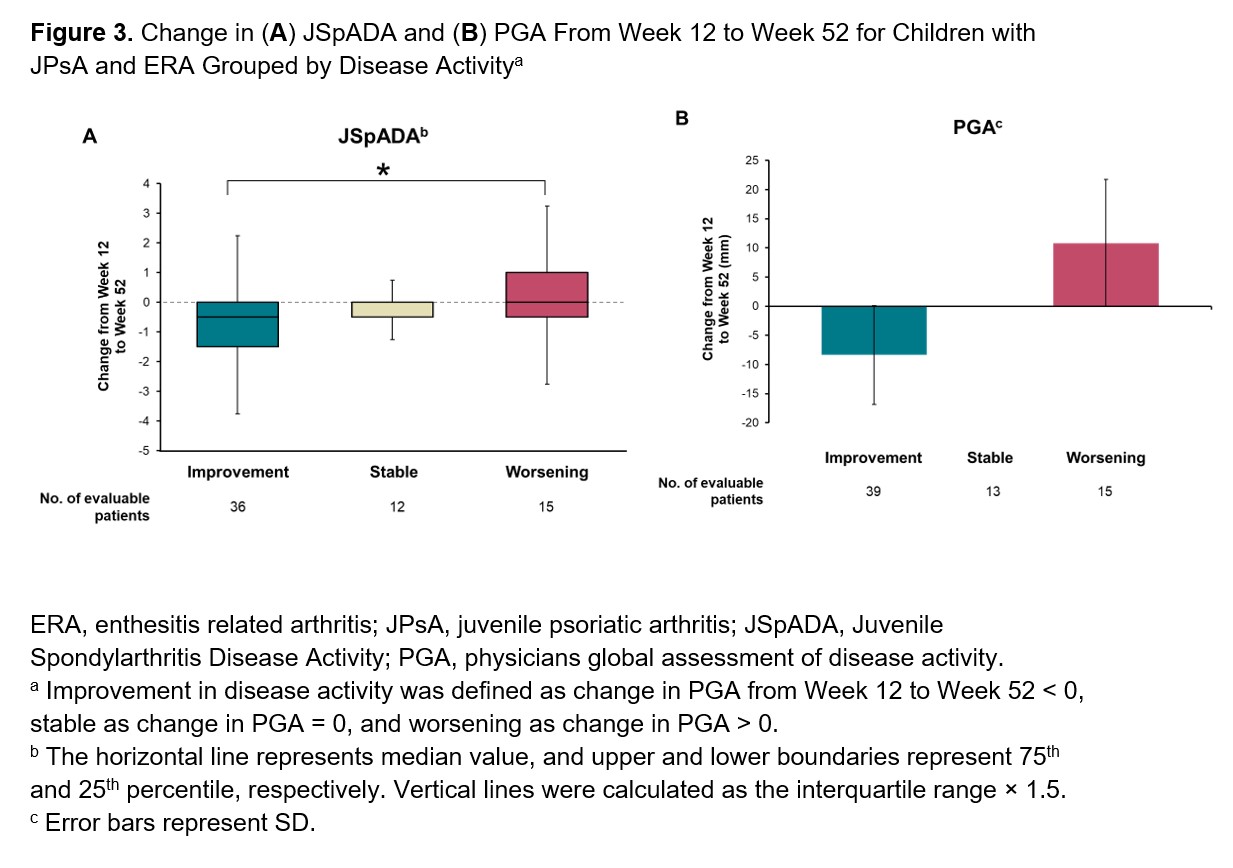
Results: Among the 86 patients included in this analysis, 52 (60.5%) had ERA and 34 (39.5%) had JPsA. At Week 12, patients with active disease had higher mean (SD) JSpADA scores than patients with inactive disease (1.8 [1.3] vs 0.5 [0.8]; P< .0001; Figure 1). JSpADA scores showed moderate to good correlation with JADAS-10, cJADAS-10, and PGA scores at Week 12 (Spearman’s correlation coefficient; range, 0.62-0.72; Figure 2). Patients with improvements in disease activity from Weeks 12 to 52 as determined by change in PGA scores had greater improvements in mean JSpADA scores over the same time period than patients with worsening in disease activity (−0.8 [1.1] vs 0.4 [1.0]; P< .001; Figure 3). Patients with stable disease from Weeks 12 to 52 had minimal change in JSpADA (−0.1 [0.48]).
Conclusion: JSpADA discriminated between active and inactive disease, correlated with other validated disease activity measures, and responded to changes in clinical disease activity in children with ERA and JPsA in the prospective JUNIPERA study.
To cite this abstract in AMA style:
Weiss P, Ruperto N, Quebe-Fehling E, Shew A, Pricop L, Pieterse C, Brunner H. Validation of the Juvenile Spondyloarthritis Disease Activity Index in a Prospective Clinical Trial Setting [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/validation-of-the-juvenile-spondyloarthritis-disease-activity-index-in-a-prospective-clinical-trial-setting/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validation-of-the-juvenile-spondyloarthritis-disease-activity-index-in-a-prospective-clinical-trial-setting/