Session Information
Session Type: Poster Session B
Session Time: 5:00PM-6:00PM
Background/Purpose: Children with rheumatic disease frequently require management with immune suppressing medications. The benefits of these interventions often outweigh the risks, however serious adverse events (SAE) will occur in a subset of patients. Accurately capturing SAEs is a cornerstone of pharmacovigilance. The Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry is a multicenter collaboration to maintain such data. It is unknown if underreporting exists in registry site self-reported event data in the CARRA registry. This study aims to identify novel ways to identify SAEs using a study sites electronic health record (EHR) tools and compare to the Registry.
Methods: Five CARRA sites each performed a chart review of approximately thirty randomly selected children enrolled in the Registry. The most recent 24 months, or the duration of registry enrollment if 24 months, were examined. SAEs identified using a variety of methods in the electronic health records including creating filters, keyword search, manually reviewing all patient encounters, and reviewing outside medical records when available. The variables were collected in a standardized template and aggregate data were examined. Admissions prior to CARRA enrollment were excluded. Routine hospitalization for medication infusion, sedation for diagnostic testing, sedation for joint injections, or admissions unrelated to their primary diagnosis were excluded. The CARRA database was then reviewed for the same patients to collect reported SAEs adverse events over the same time frame.
Results: A total of 156 patients were included for analysis with 353 patient years of observation. Although Admission filters were available, 30% of identified admission included non-event admissions, such as sedation or a scheduled procedure. Although some clinic note templates were standardized to capture event data, none were available in a discrete, reportable field. Only one site leveraged pre-visit patient-questionnaires to directly ask patients these data . We identified 77 hospitalizations with 14 directly related to infection in our patient sample (Table 1). A rate of 3.96 infection related hospitalizations per 100 patient years was calculated compared to 1.98 infection related hospitalizations reported.
Conclusion: We identified significant underreporting of SAE in a random sample of patients enrolled in the CARRA from five participating sites utilizing tools in the electronic health record. This may lead to mischaracterization of the risks of treatment in this patient population. Future efforts leveraging existing tools in the electronic health record may streamline the reporting process and minimize the prevalence of SAE under reporting.
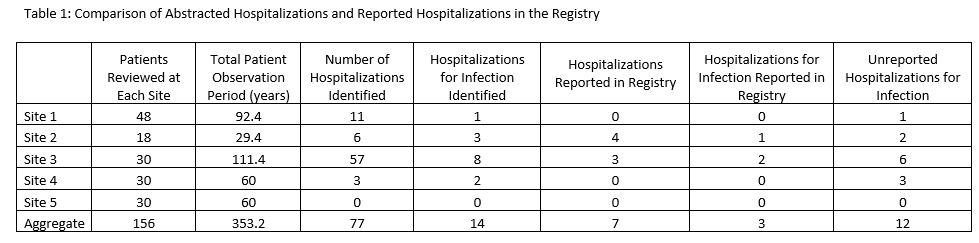 Comparison of Abstracted Hospitalizations and Reported Hospitalizations in the Registry
Comparison of Abstracted Hospitalizations and Reported Hospitalizations in the Registry
To cite this abstract in AMA style:
Basiaga M, Pooni R, Pinotti C, Buckley L, Taxter A, Investigators C. Validation of Serious Adverse Event Reporting in a Multicenter Registry [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 4). https://acrabstracts.org/abstract/validation-of-serious-adverse-event-reporting-in-a-multicenter-registry/. Accessed .« Back to 2023 Pediatric Rheumatology Symposium
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validation-of-serious-adverse-event-reporting-in-a-multicenter-registry/
