Session Information
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: New user designs are standard in many pharmacoepidemiology studies, but a first prescription appearance in electronic health record (EHR) data may not always represent a new medication start. The optimal implementation of new user designs in single-specialty EHR data is unknown.
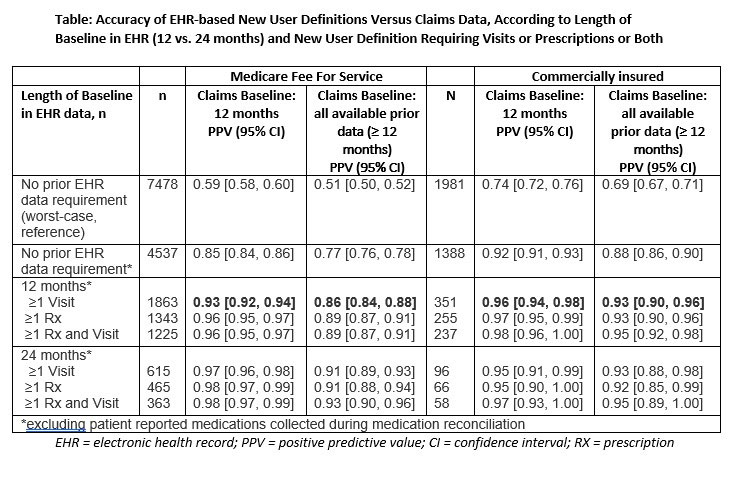
Methods: We identified patients with new prescriptions for biologics or janus kinase inhibitors (JAKi) for RA or spondyloarthritis ordered by rheumatologists in the Illumination Health Real-World Evidence Platform (EHR data) linked to either fee-for-service Medicare or commercial health plan data (claims data). The first prescription observed in the EHR data for each medication anchored the index date. Drugs reported by the patient as part of medication reconciliation (one of the most adopted quality measures, MIPS #130) that preceded the first observed prescription in the EHR data were excluded. We tested 6 new user definitions that required a rheumatologist office visit, any prescription in the EHR data, or both, occurring in the EHR ≥ 12, or ≥ 24 months prior to index. We evaluated the positive predictive values (PPV) of the EHR new user definitions using an absence of previous prescription fills in claims data as the gold standard for determining whether prescriptions were truly new medication starts, using a 12-month baseline period and also an “all-available” lookback period prior to index (minimum of 12 months). For reference purposes, we also evaluated the amount of misclassification occurring if patient reported medications were not excluded.
Results: There were 7,478 medication initiations in patients linked to Medicare (mean[SD] patient age 69.6[10.4] years, 78.7% women) and 1,981 in patients linked to commercial claims (mean[SD] patient age 64.9[12.6] years, 78.6% women). Most patients in the cohort (81% Medicare, 75% Commercial) had RA. In Medicare and commercial health plan data, the mean(SD) years of prior claims data was 5.8(3.7) (Medicare) and 4.8(3.2) (commercial), and patients had previously filled a mean(SD) of 0.9(1.1) and 0.9(1.0) biologics or tsDMARDs, respectively. The PPVs of the 6 new user definitions ranged from 85-98% vs a fixed 12m claims-based gold standard, and 86-93% vs an “all-available data” claims-based gold standard in Medicare-linked patients (Table). PPVs ranged from 95-97% (vs fixed 12m claims-based gold standard) and 92-95% (vs all-available claims-based gold standard) in commercially insured patients. Compared to an EHR new user definition that only required a rheumatologist visit ≥12 months before new medication use, requiring both visits and medications, or using 24-month EHR baseline, increased PPVs by only 1-3% in both datasets with appreciable loss in sample size.
Conclusion: A new user definition for EHR data that requires ≥1 rheumatologist visit at least 12 months before first drug mention is a valid approach to identify new medication users. Although less accurate, excluding medications based only on patient-reported medication information collected during medication reconciliation also showed good performance with increased sample size.
To cite this abstract in AMA style:
Curtis J, Su Y, george M, Clinton C, Stewart P, Buekelman T, Xie F. Validation of New Medication User Definitions in Single-Specialty EHR Data [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/validation-of-new-medication-user-definitions-in-single-specialty-ehr-data/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validation-of-new-medication-user-definitions-in-single-specialty-ehr-data/