Session Information
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Systemic lupus erythematosus (SLE) is the archetypic systemic autoimmune disease, for which there is mounting evidence for roles for intestinal bacteria in the development of the systemic autoimmune responses, inflammation, and tissue injury. Whereas Lupus nephritis (LN) is amongst the most serious and potentially life-threatening complications of SLE, earlier diagnosis would speed clinical decision-making and initiation of definitive therapy. To characterize the interrelationships between Lupus host immunity and a candidate gut microbiome pathobiont, we have recently described an assay for detection of serum IgG responses to the commensal anaerobe, Ruminococcus gnavus (RG), for identification of individuals with active LN in three US adult Lupus cohort. In the current studies, we further validated this assay and investigated its potential utility in a Swedish cohort.
Methods: Patients were recruited and characterized at the Karolinska Hospital, based on ACR criteria, with 38 LN confirmed by renal biopsy, 38 non-renal SLE patients, with 37 age- gender- ethnicity- matched unaffected population (Healthy) controls, 20 ANCA-associated vasculitis (AAV) and 14 IgA Nephropathy (IgAN) patients, based on renal biopsy and/or standard clinical criteria. At our NYU lab, serologic studies were performed with a previously described custom bead-based serologic assay with nuclease-treated RG2 extract and control analytes.
Results: LN patients had significantly higher levels of IgG anti-RG2 than Healthy subjects (p< 0.0001,) other non-renal Lupus patients (p=0.0002), or patients with IgAN (p< 0.0001) or AAV (p< 0.0001)(Wilcoxon rank-sum). There was a modest difference between non-renal Lupus patients, and Healthy subjects (p=0.0283), but no difference between Healthy with IgAN (p=0.273) or AAV (p=0.433). ROC analysis, when LN were compared to Healthy subjects gave an AUC of 0.863 and a Likelihood ratio (LR) of 5.6 at a cut-off of the mean+1 SD for control subjects (p< 0.0001); the LR was 5.8 for a cut-off of a mean+2SD (p< 0.0062), and LR of 7.8 for a cut-off of a mean+3SD (p=0.0284). For LN compared to the disease controls (AAV and IgAN) there was an AUC of 0.828 with a LR of 2.64 with a cut-off of a mean+1SD for disease controls (p=0.01), and the LR of 5.4 with a disease control mean+2SD (p=0.0071)(Figure 1A&B).
Conclusion: These findings further validate that serum IgG anti-RG2 levels provide a robust biomarker for the identification of LN, unaffected by gender and age. Importantly, the assay displayed attractive performance characteristics for discrimination of LN patients from population controls as well as those with pathologic proteinuria due to other forms of immune glomerulopathy (i.e., AAV and IgAN). Our findings also provide circumstantial evidence of a possible role for the R. gnavus pathobiont in patients in Sweden, suggesting this pathobiont may be involved in LN at sites geographically distant from the localities in which this association was first identified.
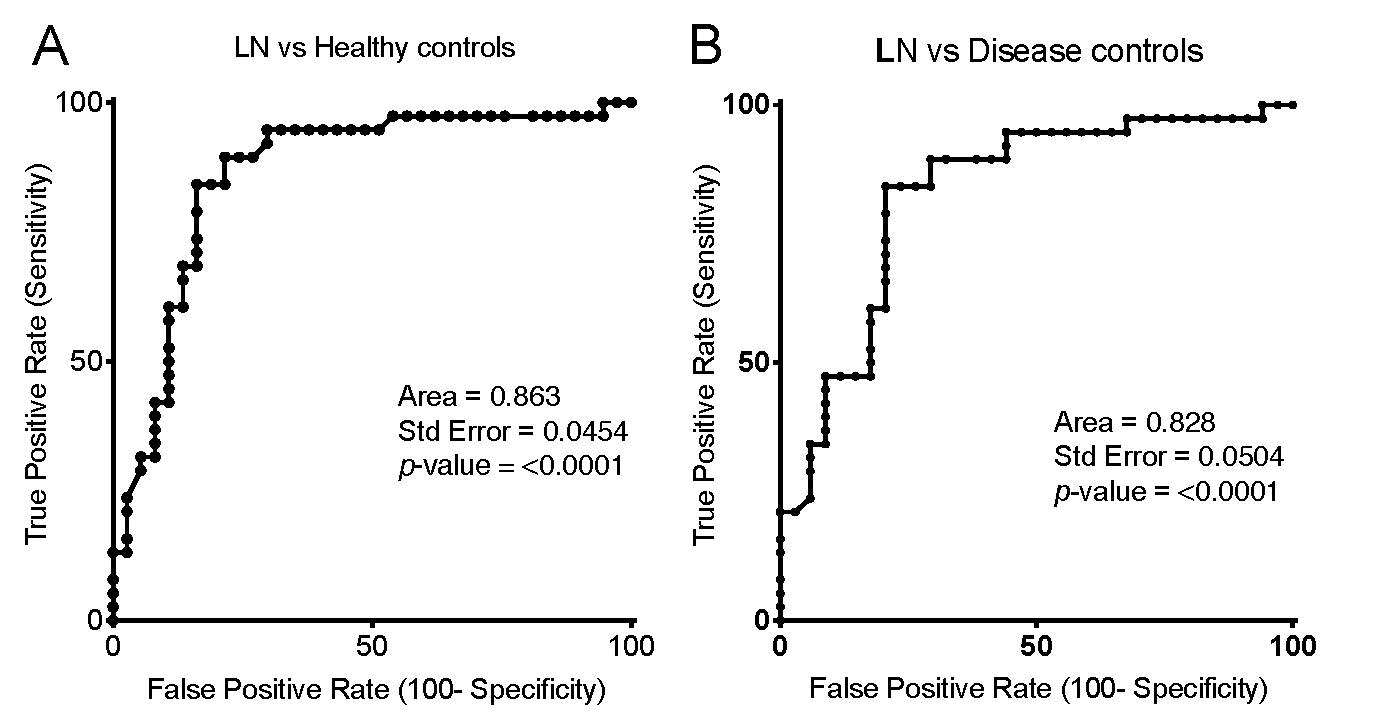
Figure A&B for ACR abstract 06012019
To cite this abstract in AMA style:
Silverman G, Azzouz D, Grönwall C, Gunnarsson I, Svenungsson E. Validation of a Serologic Antibody Biomarker Against a Candidate Gut Pathobiont for the Diagnosis of Lupus Nephritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/validation-of-a-serologic-antibody-biomarker-against-a-candidate-gut-pathobiont-for-the-diagnosis-of-lupus-nephritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validation-of-a-serologic-antibody-biomarker-against-a-candidate-gut-pathobiont-for-the-diagnosis-of-lupus-nephritis/
