Session Information
Date: Monday, November 8, 2021
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster III: Outcomes (1257–1303)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Development of effective new Systemic Lupus Erythematosus (SLE) treatments requires a validated responder index responsive to clinically meaningful change and relevant to clinical practice. To address this challenge, we have recently developed a new Lupus Multivariable Outcome Score (LuMOS) to optimize discrimination between outcomes of actively treated patients versus those randomized to placebo [1]. We now report on external validation of LuMOS in two independent SLE clinical trials.
Methods: Validation was carried out in the Illuminate trials used to evaluate tabalumab (TB) in SLE. All participants in both Illuminate 1 and 2 clinical trials met the ACR classification criteria for SLE and all were included in our analyses. To adapt LuMOS for use with laboratory results assessed on different platforms than used in the trials of belimumab employed to generate the original LuMOS outcome score [1], we calculated a standardized score using z-score transformation. For validation, in each of the Illuminate trials, we calculated LuMOS scores at week 52 for all participants receiving either placebo or one of 2 dosage regimens of TB. Cohen D Effect Size (ES), with 95% confidence intervals (CI), assessed the ability of LuMOS to discriminate between outcomes in active treatment groups versus placebo, and compared it with SRI-5 responder index used in the trials.
Results: LuMOS using standardized z-score transformed laboratory data worked comparably to the original LuMOS model in the original data from the belimumab trials and was then applied to the Illuminate data sets. LuMOS-based ES for both TB groups in both trials indicated highly significant (p< 0.0001), moderately strong treatment effects (ES >0.4), in contrast to weak effects (ES< 0.25) based on SRI-5, that were statistically non-significant for Illuminate-1, as reflected by the 95% CI’s that included 0 (Table).
Conclusion: LuMOS improves the ability to detect statistically significant treatment effects. Further validation in SLE trials of non-B cell directed treatments is necessary to document utility as an outcome measure independent of drug mechanism.
[1] Abrahamowicz et al. Arthritis Rheumatol. 2018;70:1450-58.
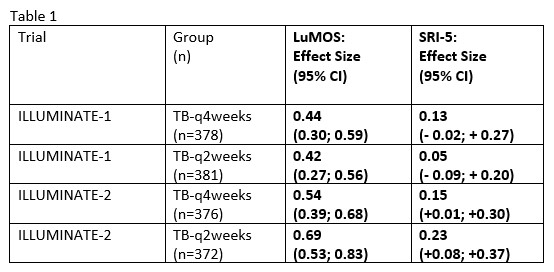 Comparison of Effect Sizes of LuMOS vs. SRI-5 for discrimination between TB-treated and placebo groups n Illuminate_1/_2 trials
Comparison of Effect Sizes of LuMOS vs. SRI-5 for discrimination between TB-treated and placebo groups n Illuminate_1/_2 trials
To cite this abstract in AMA style:
Abrahamowicz M, Lipsky P. Validation of a Novel Lupus Multivariable Outcome Score as an Outcome Measure for Systemtic Lupus Erythematosus Trials [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/validation-of-a-novel-lupus-multivariable-outcome-score-as-an-outcome-measure-for-systemtic-lupus-erythematosus-trials/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validation-of-a-novel-lupus-multivariable-outcome-score-as-an-outcome-measure-for-systemtic-lupus-erythematosus-trials/
