Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Osteoarthritis (OA) causes joint pain, stiffness, swelling, and functional impairment. Pain signals reach the brain, influencing inflammatory and immune responses through the parasympathetic efferent vagus nerve. Here we test if vagal nerve stimulation (VNS) improves OA pain, inflammation, and progression in two mouse models: destabilization of the medial meniscus (DMM), a slow OA model, and forced tibial compression anterior cruciate ligament (ACL)-rupture, a rapid OA model.
Methods: In the DMM model, 16-week(wks)-old female C57BL/6 mice (n=20) had DMM surgery on the right knee. After 4 wks, they were treated with 8 wks of VNS or SHAM stimulation for 10 minutes/day, 5 days/wk. Evoked mechanical hyperalgesia with pressure algometry and weight bearing pain responses were assessed at pre-VNS, and after 4 and 8 wks of VNS or SHAM stimulation. In the second model, 16-wk-old female C57BL/6 mice (n=20) underwent ACL rupture followed by 2 wks of VNS or SHAM stimulation beginning one day after injury to assess the utility of VNS on acute joint pain. Weight-bearing, evoked hyperalgesia, and allodynia using Von Frey filaments, were measured after 2 wks of VNS or SHAM stimulation. At the endpoint of DMM (8 wk treatment) and ACL (2 wk treatment), serum samples were collected for cytokine analysis using a 23-plex assay, and knee joints were collected for histological examination using the OARSI grading method.
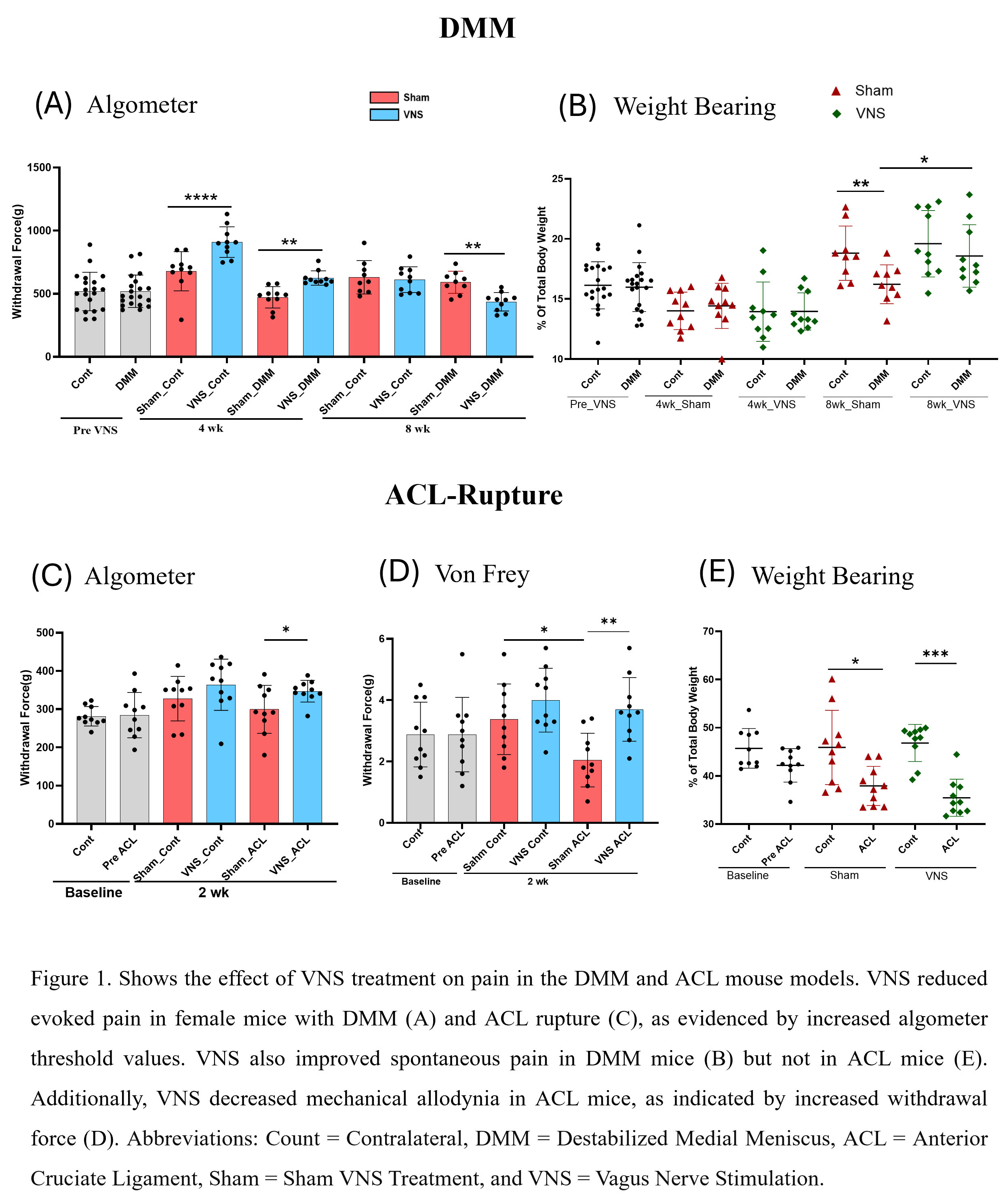
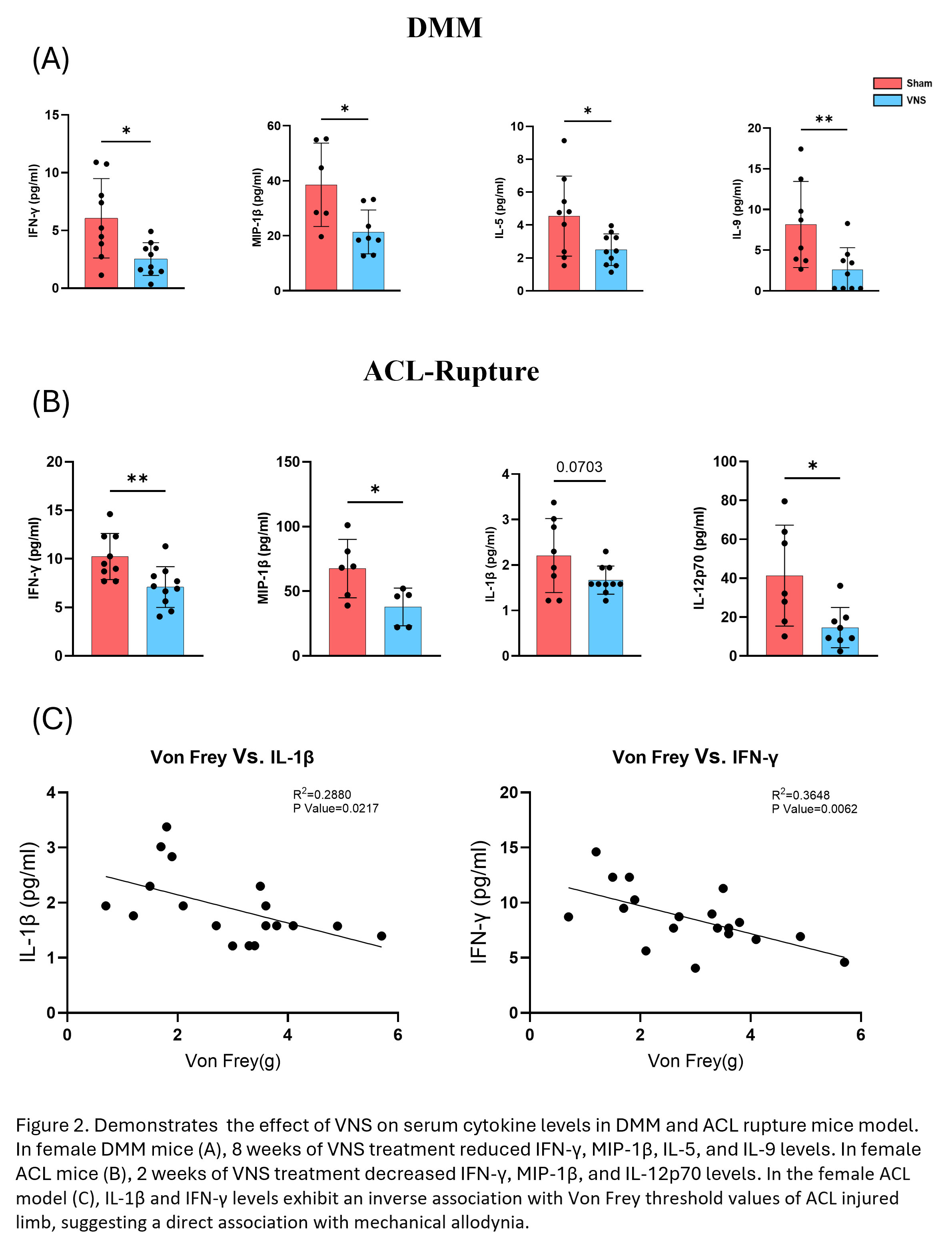
Results: In the DMM model, VNS-treated mice exhibited significant improvement in evoked hyperalgesia at 4 weeks and weight-bearing pain at 8 weeks compared to SHAM-stimulated mice. Female mice had very low OARSI scores that did not differ with treatment. VNS treatment significantly reduced serum levels of INF-γ, IL-5, IL-9 and MIP-1β. In the ACL model, 2 wks of VNS treatment improved hyperalgesia and allodynia compared to SHAM treatment. ACL rupture induced weight-bearing pain, and treatment did not modify this pain. Two wks of VNS treatment significantly reduced OARSI and posterior zone OA scores in the ACL-injured lateral tibia compartment compared to SHAM treatment. Like the DMM results, VNS treatment reduced INF-γ and MIP-1β levels and reduced IL-12p70 and IL-1β levels. After ACL rupture, OARSI scores were significantly reduced with VNS treatment in the lateral compartment and the posterior zone of the tibia.
Conclusion: VNS reduced pain and systemic inflammation in two OA mouse models in female mice. VNS improved mechanical hyperalgesia in DMM and ACL rupture, mechanical allodynia in ACL rupture, weight-bearing pain in DMM, and osteoarthritic damage (OA scores) after ACL rupture. Changes in serum cytokines with VNS treatment showed a consistent reduction in INF-γ and MIP-1β between the slow DMM model and the faster ACL rupture model, suggesting that these cytokines are linked to pain responses in both models. Further studies are required to understand the mechanisms of pain reduction and protection of joint destruction by VNS treatment. These positive results of VNS treatment for OA pain support human clinical studies testing VNS treatment for chronic OA pain and acute ACL injury pain and prevention of joint remodeling.
To cite this abstract in AMA style:
Yadav S, Griffin T, Jeffries M, Stavrakis S, Humphrey M. Vagus Nerve Stimulation: A Promising Approach to Alleviate Pain and Systemic Inflammation in Slow and Rapid Models of Osteoarthritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/vagus-nerve-stimulation-a-promising-approach-to-alleviate-pain-and-systemic-inflammation-in-slow-and-rapid-models-of-osteoarthritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/vagus-nerve-stimulation-a-promising-approach-to-alleviate-pain-and-systemic-inflammation-in-slow-and-rapid-models-of-osteoarthritis/