Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Uveitis is a well-known non-musculoskeletal manifestation of spondyloarthropathies. Patients typically suffer from blurred vision, pain, and photophobia and have an increased risk of ocular complications. Prevalence varies greatly, as uveitis occurs in up to 50% of patients with ankylosing spondylitis (AS) and in approximately 7% of patients with psoriatic arthritis (PsA).1 The German non-interventional study AQUILA provides real-world data on the development of uveitis in patients with AS or PsA under treatment with secukinumab, a fully human monoclonal antibody that selectively inhibits interleukin-17A. The aim of this interim analysis is to compare baseline (BL) characteristics depending on the presence of uveitis, as well as frequencies and course of uveitis during the study.
Methods: AQUILA is an ongoing, multi-center, non-interventional study including up to 3000 secukinumab treated patients with active AS or PsA. Patients were observed from BL up to week 52 according to clinical routine. Real-world data was assessed prospectively and analyzed as observed. Uveitis and its severity (mild, moderate, severe) was determined by the treating rheumatologist at BL and subsequent study visits. This interim analysis focuses on the subgroups of patients with or without uveitis at BL.
Results: At BL, 668 AS and 1216 PsA patients were included with information on uveitis status available: Prevalence of uveitis at BL was 6.3% (n=42) in AS and 1.7% (n=21) in PsA patients. Demographic data (Table 1) showed that the percentage of males was higher in AS patients with uveitis (78.6%) compared to those without (58.5%). PsA patients did not show this distribution as the percentage of males was lower in patients with uveitis (38.1% versus 41.7%). For both AS and PsA patients, the proportion of obesity (i.e. BMI >30 kg/m2) was higher in patients with uveitis. One AS patient was included with a moderate uveitis which resolved and came back later with the same severity (uveitis status of this patient was considered as unchanged compared to BL; Figure 1). In all other cases, uveitis status improved or remained unchanged (Figure 1). Furthermore, most of the patients with uveitis remained on secukinumab treatment up to 52 weeks (AS: 61.9%, n=26; PsA: 81.0%, n=17). Both AS and PsA patients with uveitis, had a numerically higher percentage of pretreatment with bDMARDs (biological disease-modifying antirheumatic drugs). In addition, more patients with uveitis received concomitant corticosteroids compared to patients without uveitis throughout 52 weeks observational period (Table 1). Regarding patients without uveitis at BL, 3 patients (1 out of 626 with AS and 2 out of 1195 with PsA) developed new onset uveitis during the study.
Conclusion: In a real-world setting, the number of new onset uveitis under treatment with secukinumab was low. In most patients with uveitis at BL, uveitis status remained unchanged or even improved throughout 52 weeks observational period. Altogether, real-world data of this interim analysis are in line with those of Phase 3 studies and show that secukinumab did not increase the risk of uveitis.
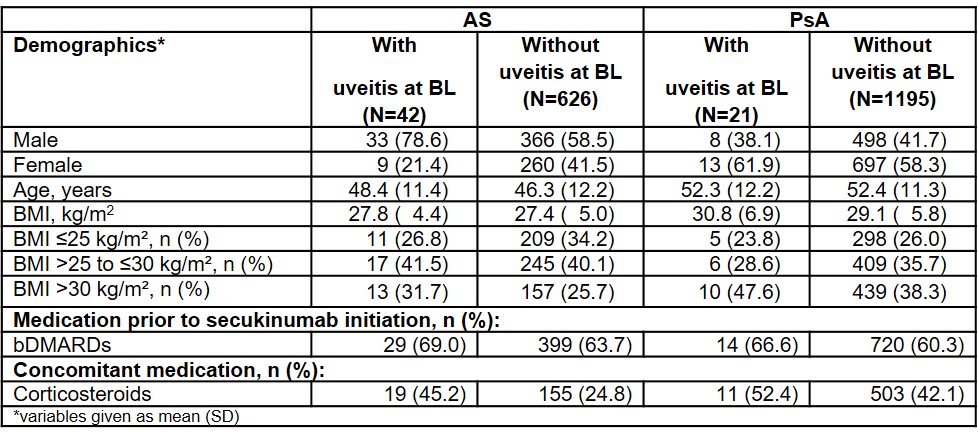 Table 1: Overview of BL characteristics depending on the presence of uveitis
Table 1: Overview of BL characteristics depending on the presence of uveitis
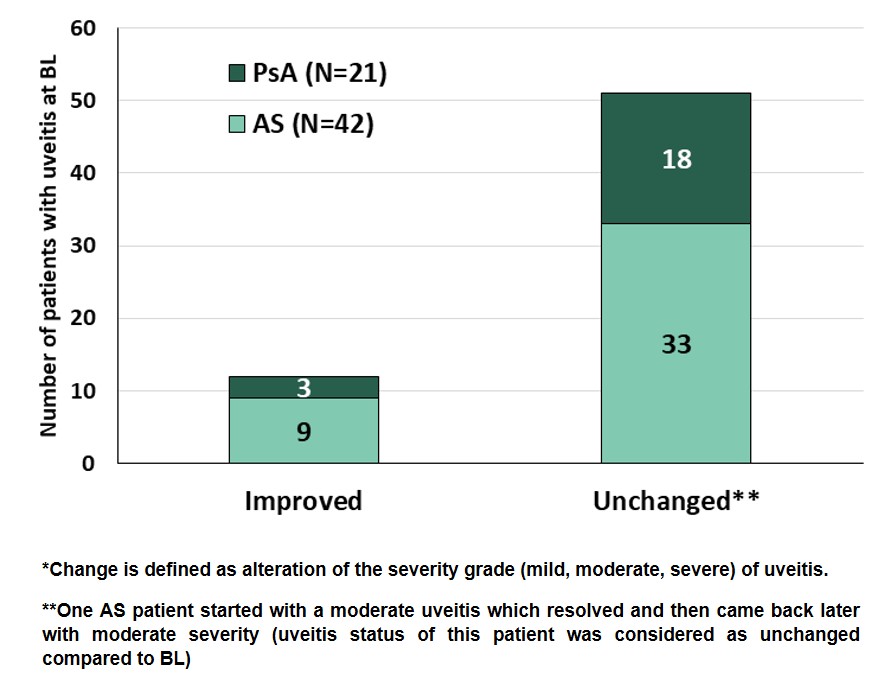 Figure 1: Change of uveitis status* within 52 weeks of observational period
Figure 1: Change of uveitis status* within 52 weeks of observational period
To cite this abstract in AMA style:
Kiltz U, Brandt-Jrgens J, Kästner P, Riechers E, Peterlik D, Boas A, Tony H. Uveitis Status in Patients with Ankylosing Spondylitis or Psoriatic Arthritis Under Secukinumab Treatment – Real World Data from a German Observational Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/uveitis-status-in-patients-with-ankylosing-spondylitis-or-psoriatic-arthritis-under-secukinumab-treatment-real-world-data-from-a-german-observational-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/uveitis-status-in-patients-with-ankylosing-spondylitis-or-psoriatic-arthritis-under-secukinumab-treatment-real-world-data-from-a-german-observational-study/
