Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Lupus nephritis (LN) affects up to 50% of patients with Systemic Lupus Erythematosus. Around 40% of patients will experience a subsequent renal flare in the following 3-4 years, and up to 20% will progress to end-stage renal disease within 10 to 15 years after onset. Repeat kidney biopsies (KB) performed 2 years after the last LN flare have been shown to predict subsequent renal flares and long-term renal dysfunction. However, KBs are invasive. In this study, we assessed whether five urinary biomarkers (UB), including CD163, MCP-1, Adiponectin, sVCAM-1 and PF4, that have previously been shown to discriminate between active and non-active LN patients and reflect clinical improvement, measured 2 years after a LN flare, predict subsequent LN flares and decline in kidney function over a 10-year follow-up period.
Methods: Patients who had a LN flare and stored urine 24±3 months after the LN flare were included in the study. The 5 UB levels were measured by ELISA 24±3 months after the LN flare. The following renal outcomes were then examined: 1) Time to a subsequent LN flare (defined by an increase in proteinuria of at least 1000 mg/day if the baseline was < 500 mg/day or doubling of proteinuria if the baseline was ≥500 mg/day, prompting a change in therapy) and 2) time to 30% decline in eGFR, after their 2-year urinary sample collection.
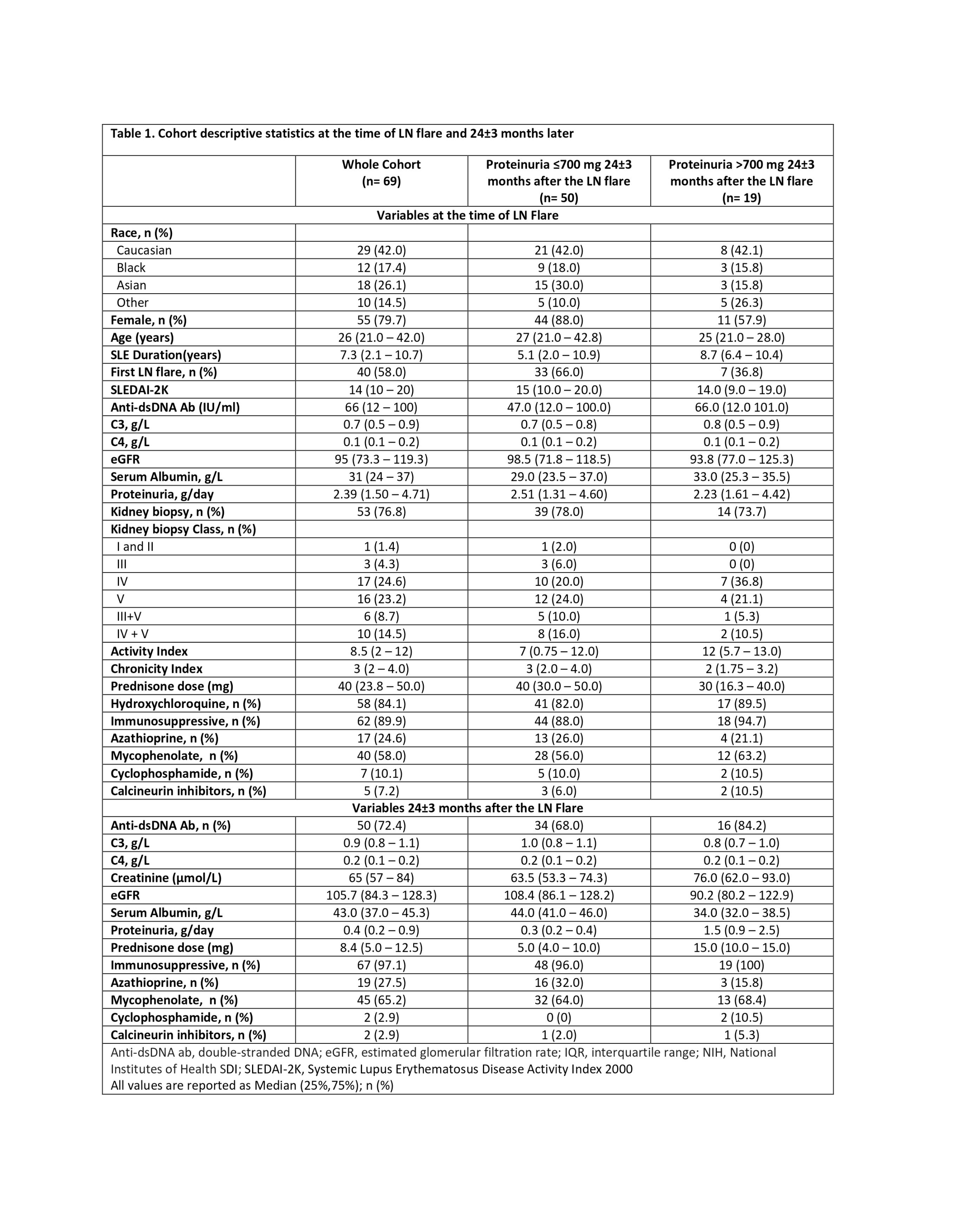
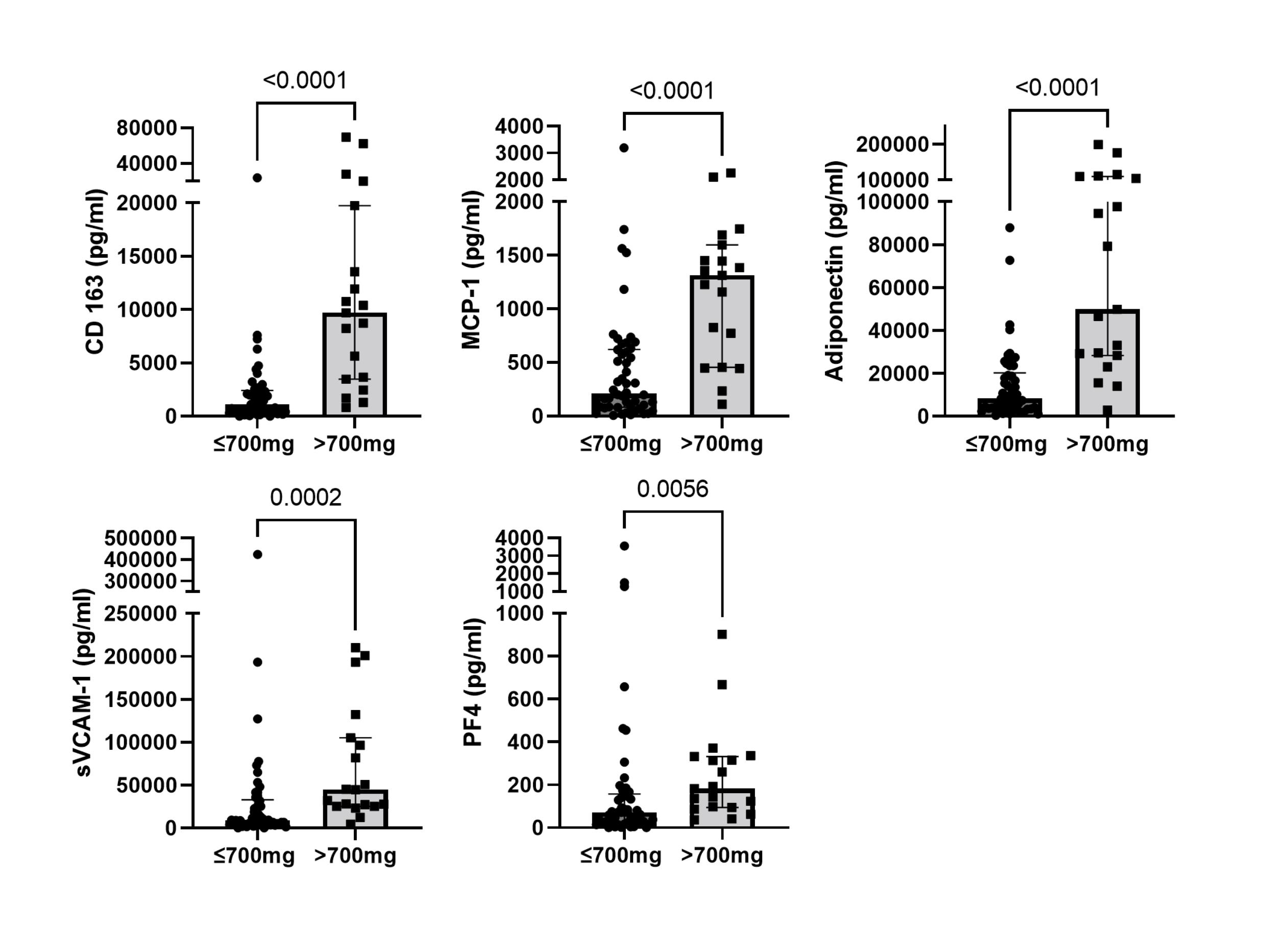
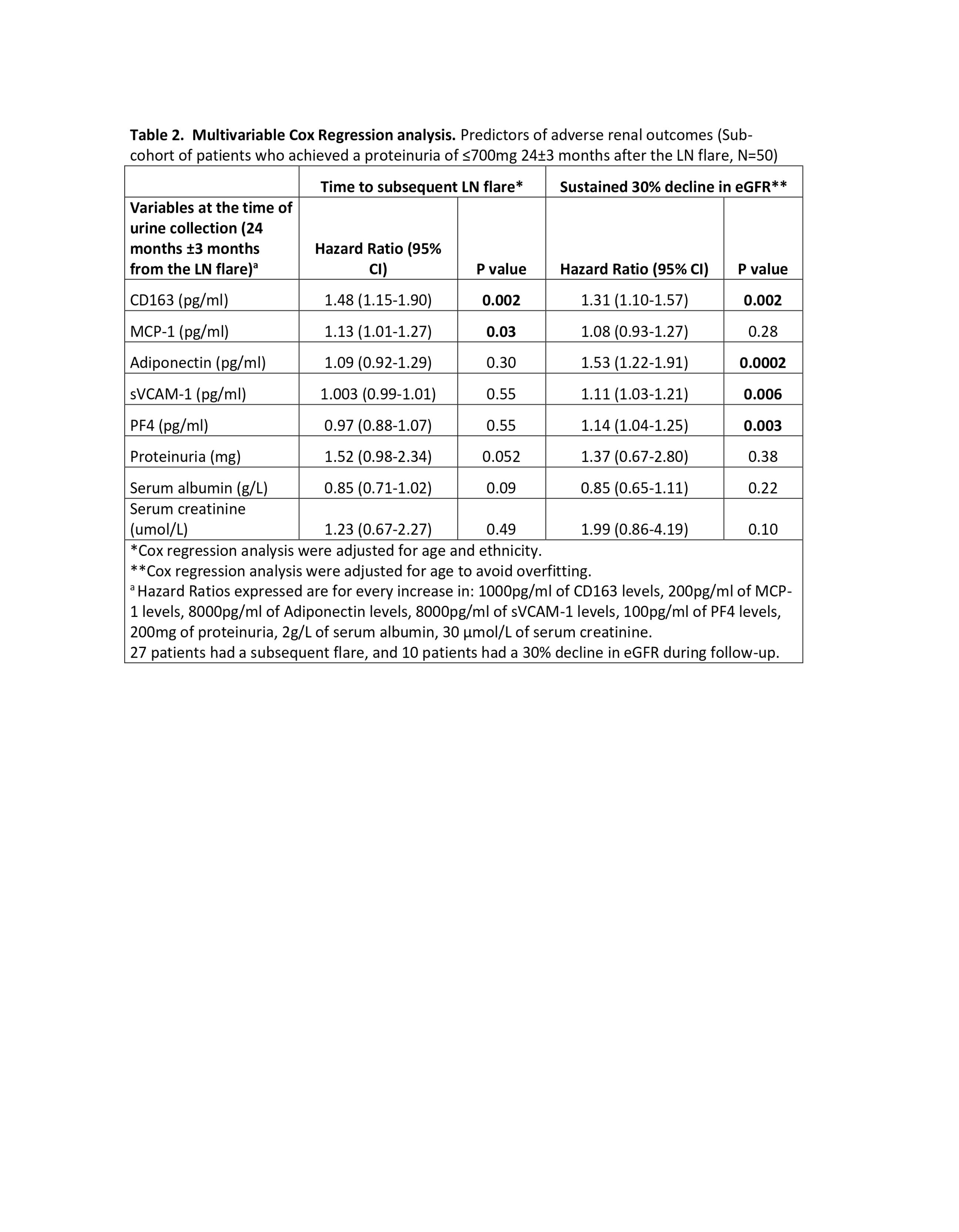
Results: Sixty-nine patients with LN were included in the study. Table 1 shows the descriptive statistics for the cohort at the time of the LN flare and 2 years after the LN flare. The median (IQR) follow-up time after their 2-year urinary sample collection was 129 (97.5-150) months. Fifty patients achieved proteinuria of ≤700mg at 2 years after the LN flare. This sub-cohort of patients had significantly lower UB levels 2 years after the LN flare compared to patients who persisted with proteinuria >700mg (Figure 1). In this sub-cohort of patients, twenty-seven (54%) experienced a subsequent LN flare with a median time to flare (IQR) of 3.5 (1.67-6.87) years, and 10 (20%) had a 30% decline in eGFR at a median time of 4.38 (3.73-5.33) years after their 2-year urinary sample collection. After adjusting for age and race, elevated levels of MCP-1 (HR 1.13 (1.01-1.27), p=0.03) and CD163 (HR 1.48 (1.15-1.90), p=0.002) predicted a subsequent LN flare. While CD163 (HR 1.31 (1.10-1.57), p=0.002), Adiponectin (HR 1.53 (1.22-1.91), p=0.0002), sVCAM-1 (HR 1.11 (1.03-1.21), p=0.006), and PF4 (HR 1.14 (1.04-1.25), p=0.003) predicted a 30% decline in eGFR (Table 1), outperforming proteinuria, serum creatinine, and serum albumin, measured at the time of the UBs.
Conclusion: UB measured 2 years after an LN flare predicted long-term renal outcomes, including subsequent LN flares and decline in kidney function during follow-up.
To cite this abstract in AMA style:
Baker R, Whittall Garcia L, Kim M, Bonilla D, Urowitz M, Gladman D, Touma Z, Wither J. Utility of Urinary Biomarkers to Predict Long-term Renal Outcomes in Lupus Nephritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/utility-of-urinary-biomarkers-to-predict-long-term-renal-outcomes-in-lupus-nephritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/utility-of-urinary-biomarkers-to-predict-long-term-renal-outcomes-in-lupus-nephritis/