Session Information
Date: Saturday, November 16, 2024
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Tumor necrosis factor inhibitors (TNFi) are generally the first class of biologic disease modifying anti-rheumatic drugs (DMARDs) prescribed for rheumatoid arthritis (RA) patients with an inadequate response to conventional synthetic DMARDs. Inadequate responses to TNFi range between 30% and 82%. A molecular signature response classifier (MSRC) has been developed and previously validated to predict inadequate response to TNFi in RA patients and treatment selection informed by MSRC improves patient outcomes.
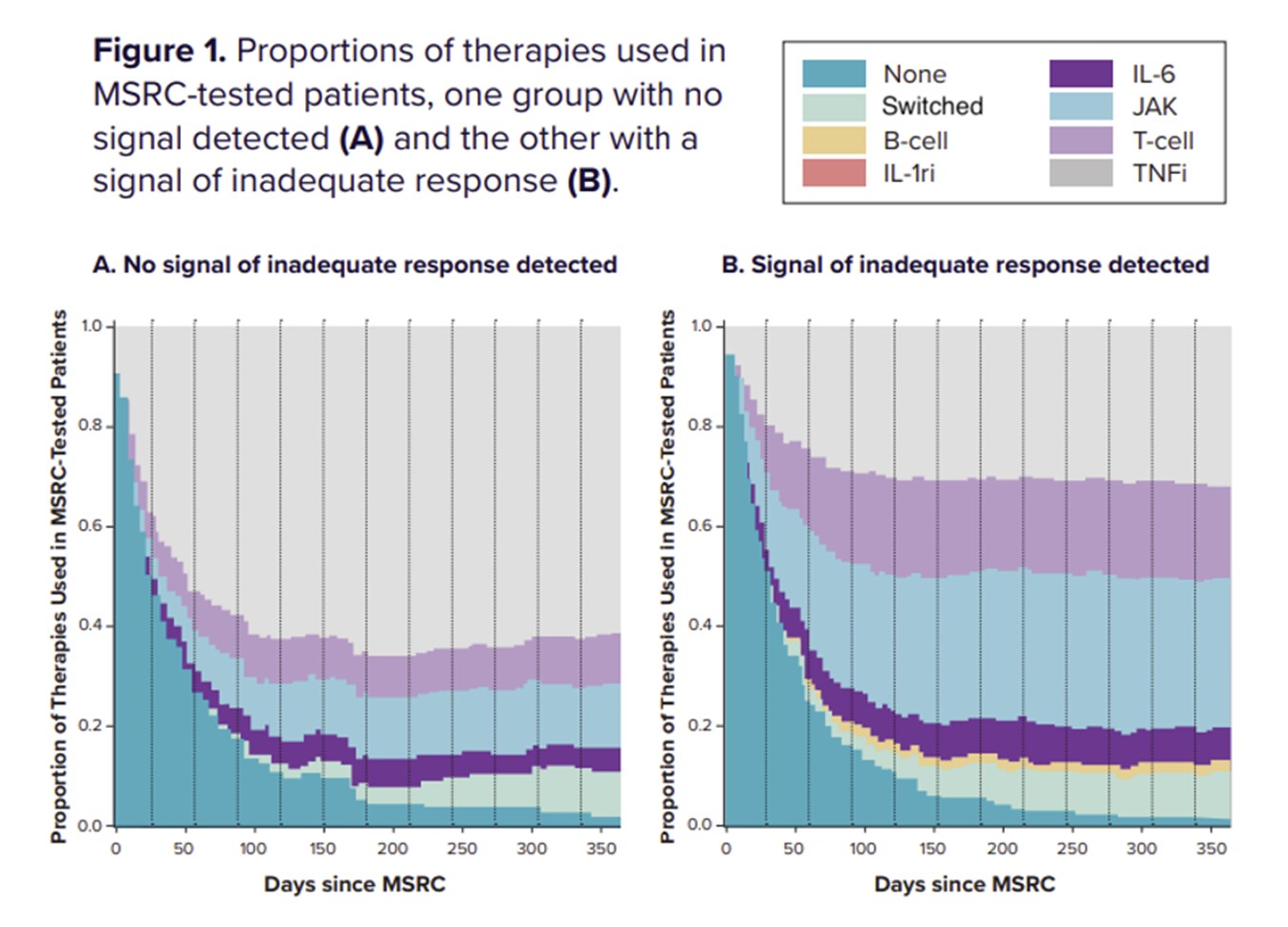
This study evaluated differences in first-line biologic treatment patterns for two cohorts of RA patients with moderate/high disease activity (CDAI >10): one with MSRC testing and the other without. The MSRC-tested cohort was stratified according to results: signal of inadequate response to TNFi and no signal of inadequate response.
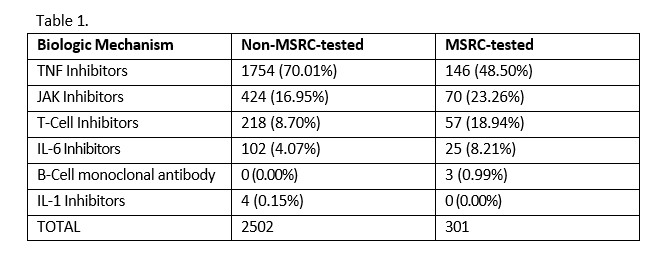
Methods: An MSRC-tested cohort (n=301) of biologic-naïve patients with CDAI >10 at therapy initiation between 2021 and 2023 was drawn from the study to Accelerate Information of Molecular Signatures in RA and U.S.-based electronic health record (EHR) databases. A second cohort of biologic-naïve patients (n=2502) with CDAI >10 without MSRC results was selected from the EHR database, prior to initiating biologic therapy in 2021. Statistical significance between two categorical variables (MSRC tested vs. not tested) and between allocated therapies by number of patients were evaluated using Pearson Chi-squared independence tests. A z-test was used for statistical evaluations of proportions of TNFi use between groups: MSRC-tested and non-MSRC-tested, and signal of inadequate response and no signal of inadequate response.
Results: TNFi were prescribed as first-line therapy in 70.1% (1754/2502) of patients not tested by MSRC, and in 48.5% (146/301) of MSRC-tested patients. In the group of MSRC-tested patients, 60.5% (182/ 301) received a signal of inadequate response and 39.5% (119/301) received no signal of inadequate response (p < 0.001). Among patients who received a signal of inadequate response, 33.5% (61/182) were prescribed TNFi, while 71.4% (85/119) of patients without a signal of inadequate response received TNFi therapy (p < 0.001). While there was a decrease in the proportion of MSRC-tested patients receiving TNFi therapy, there was a concomitant increase in the proportions of patients receiving alternative biologic and targeted therapies (p < 0.001), see Table 1.
Conclusion: In this study, an MSRC signal of inadequate response to TNFi therapies was associated with decreased utilization of TNFis and greater use of alternate therapies with differing mechanisms of action among RA patients. These data add to the growing evidence supporting the impact of MSRC testing informing management decisions, ultimately, improving patient outcomes, with ongoing studies aimed at further substantiating these findings.
To cite this abstract in AMA style:
Karpouzas G, Zhang L, Zielinski M, Cheng B, Guardiano S. UseofaMolecularSignatureResponseClassifiertoPredictInadequateResponsetoTNFiResultsinFewer Patients Prescribed TNFi [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/useofamolecularsignatureresponseclassifiertopredictinadequateresponsetotnfiresultsinfewer-patients-prescribed-tnfi/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/useofamolecularsignatureresponseclassifiertopredictinadequateresponsetotnfiresultsinfewer-patients-prescribed-tnfi/