Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Juvenile idiopathic arthritis (JIA) is the most common chronic rheumatic disease of childhood. The emergence of new biologic agents has led to changes in the prognosis and therapeutic approach for these patients. However, the use of biosimilars in adult patients diagnosed with JIA has not been well studied and more evidence is needed on the safety and persistence of these drugs in this patient population.
The aim of this study was to describe the use and assess the safety and persistence of biosimilar disease-modifying antirheumatic drugs (bsDMARDs) compared with original biologics (bDMARDs) in JIA patients older than 16 years.
Methods: Data were obtained from the nationwide prospective registry BIOBADASER (Spanish Registry of Adverse Events of Targeted Therapies in Rheumatic Diseases). All patients diagnosed with JIA and older than 16 years included in the database between 2000 and 2022 were analyzed. Due to the design of the registry, it was not possible to identify each of the JIA subgroups; therefore, we classified them into oligo/polyarticular JIA, enthesitis-related JIA, and psoriatic JIA. Adverse events were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 13.0. Proportions, means, and standard deviations (SD) were used to describe our population. Drug persistence was calculated using Kaplan-Meier survival curves (KM) until discontinuation for any reason. Incidence rates (IRs) of adverse events and 95% confidence intervals were calculated, and Poisson regression was used to estimate incidence rate ratios (IRRs), adjusting for sex, diagnosis type, age at treatment initiation (≤16 years vs >16 years), and treatment line as confounding variables.
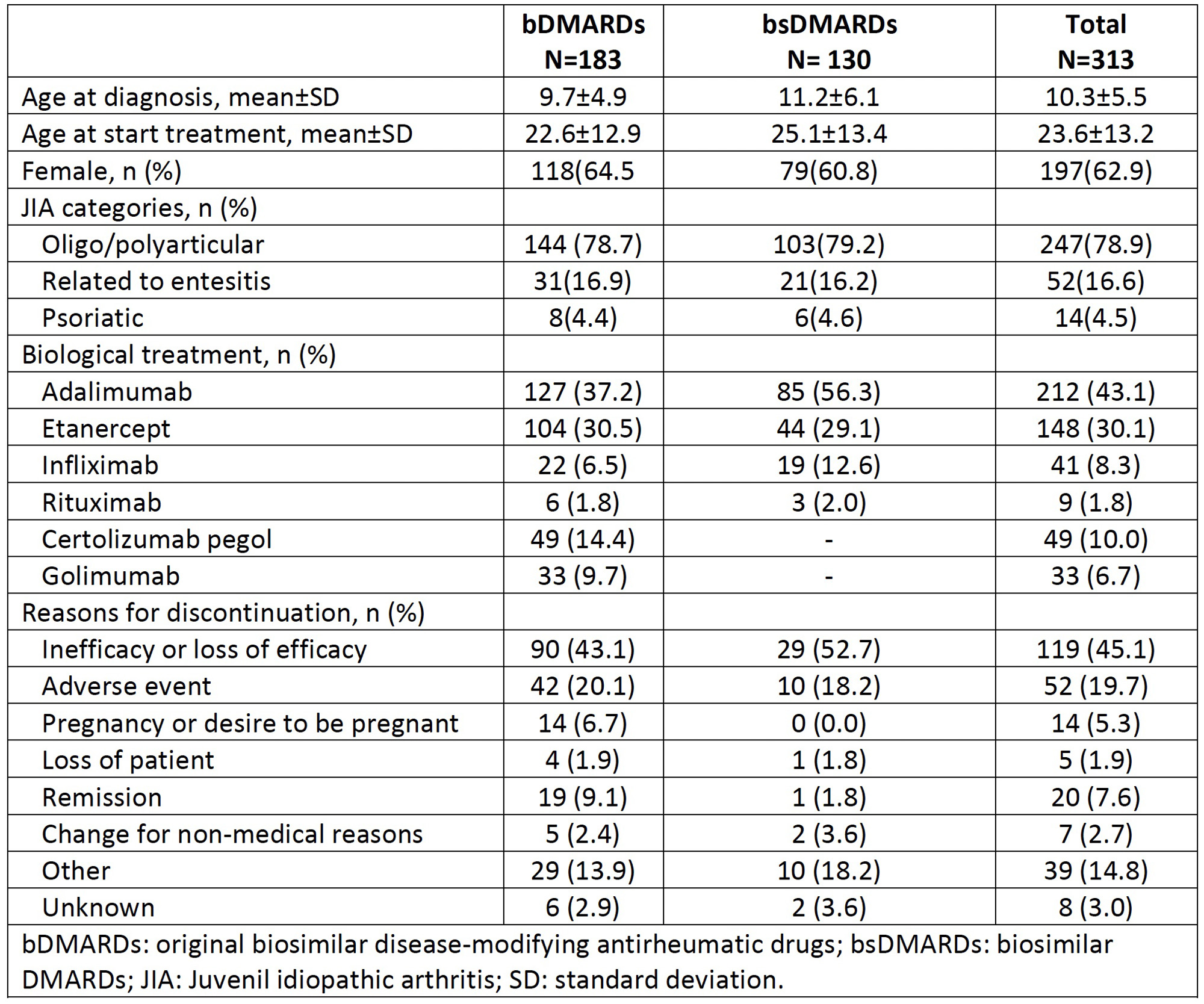
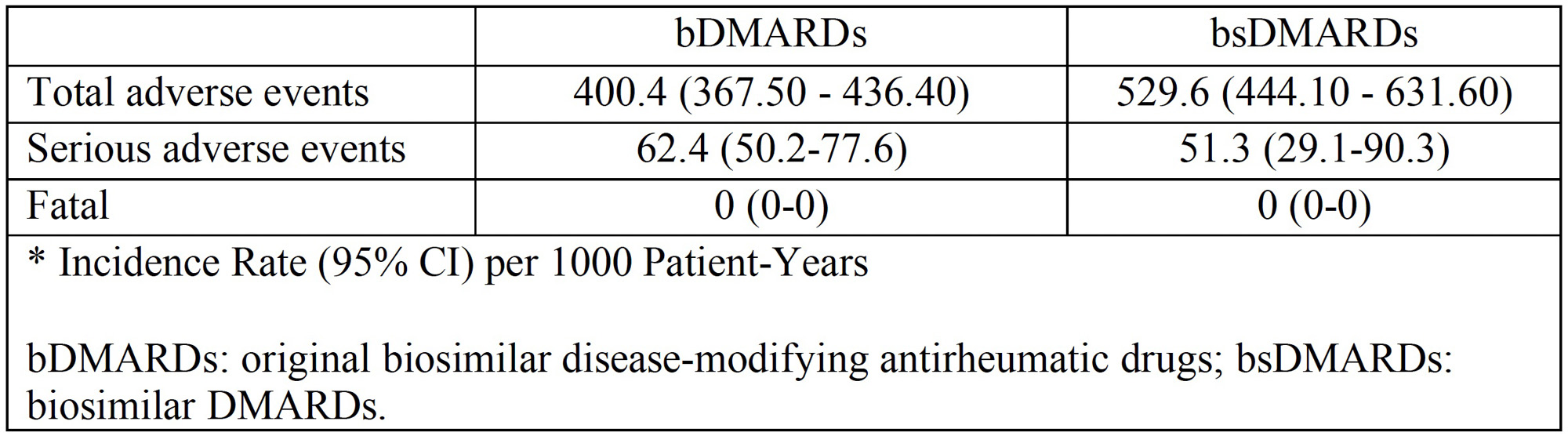
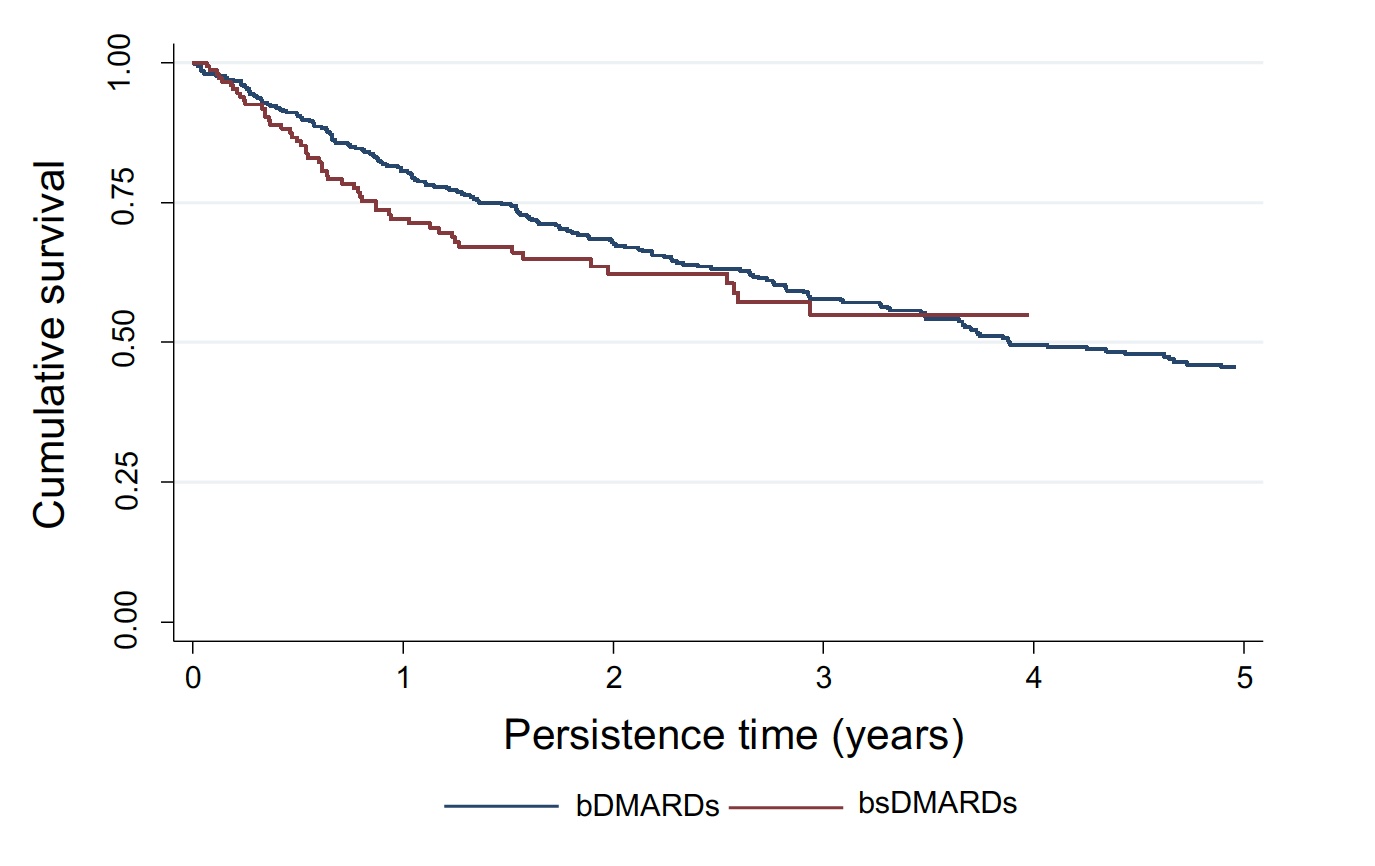
Results: From a total of 313 patients included, 130 (41.5%) patients received bsDMARDs and 183 (58.4%) treatments with bDMRAD. In the bsDMARD group, 43 patients (95.6%) use corticosteroids and 59 patients (96.7%) metotrexate concomitant.Table 1 shows the clinical characteristics, treatment and reason for discontinuation of the studied population according to treatment with bDMARD, bsDMARD and overall. Table 2 shows the IRs for adverse events according to severity. For all adverse events, IR is greater in bsDMARDs compared to bDMARDs, although the latter have a higher IR of serious adverse events. The IRR was 1.33 (95% CI 1.1-1.6) (p=0.004), thus, the risk of adverse events was greater among bsDMARD compared to the original bDMARDs. The KM figure shows that the persistence of bDMARDs and bsDMARDs was similar in the studied population (log rank: 0.78,p-value 0.377).
Conclusion: The most used bsDMARD in adult patients diagnosed with JIA was adalimumab. In this population, there were no differences in 5-year survival rates between bDMARDS and bsDMARDs in JIA adult patients, being ineffectiveness the main reason for discontinuation.
The risk of adverse events was higher in patients treated with bsDMARDs. Although this study allowed us to investigate the long-term safety of biosimilars in JIA, large registries focusing on such patients are needed to better understand rare adverse events.
To cite this abstract in AMA style:
Bethencourt J, Otero-Valera L, MANERO J, Perez-Pampin E, Pérez Vera Y, MANRIQUE S, Bustabad Reyes M, Freire González M, Ruiz-Montesinos D, Mateo Soria L, Martín Domenech R, Moreno Ramos M, Alonso F, Castrejon I. Use, Safety and Persistence of Biosimilars in Adult Patients Diagnosed with Juvenile Idiopathic Arthritis: Results from the Spanish Registry of Adverse Events of Targeted Therapies in Rheumatic Diseases (BIOBADASER) [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/use-safety-and-persistence-of-biosimilars-in-adult-patients-diagnosed-with-juvenile-idiopathic-arthritis-results-from-the-spanish-registry-of-adverse-events-of-targeted-therapies-in-rheumatic-diseas/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/use-safety-and-persistence-of-biosimilars-in-adult-patients-diagnosed-with-juvenile-idiopathic-arthritis-results-from-the-spanish-registry-of-adverse-events-of-targeted-therapies-in-rheumatic-diseas/