Session Information
Date: Sunday, November 7, 2021
Title: Measures & Measurement of Healthcare Quality Poster (0623–0659)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Intravenous immunoglobulin (IVIg) is used in several systemic rheumatic diseases due to postulated immunomodulatory properties. However, IVIg is a scarce and costly resource and poses a risk of adverse events (AEs). We evaluated the safety, effectiveness, and procurement cost of IVIg in an ambulatory rheumatic disease sample.
Methods: Using our centre’s blood bank records, we identified consecutive tertiary clinic patients receiving IVIg for rheumatic disease between January 2015 and September 2020. We performed retrospective chart reviews of all clinical encounters, from IVIg initiation until 3 months following the last infusion. We evaluated demographic and disease characteristics and clinical effectiveness of IVIg, based on the treating clinicians’ assessments 3 months after IVIg initiation and 3 months after cessation. Potential AEs and their severity documented in clinical encounter notes were adjudicated by two independent physicians using pre-established rating scales. An AE was classified as related to IVIg if there was >50% confidence of an IVIg-related adverse event. Finally, we determined if appropriate (ideal body weight-based) dosing was used and estimated the yearly procurement costs (CAN$).
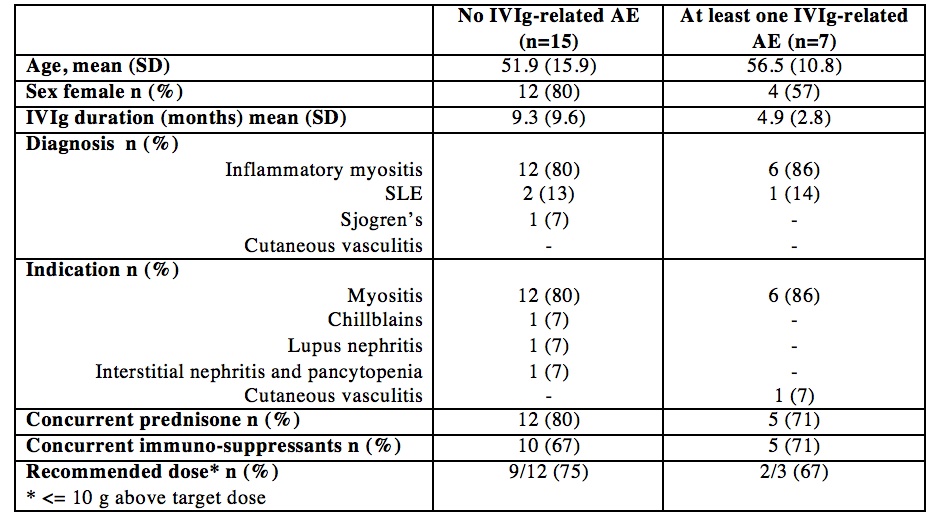
Results: Of 25 patients receiving IVIg for rheumatic disease over the study period, 22 had sufficient clinical records to be included. Mean age was 53 years (range 19-74) and 16 (72%) were women. The treatment indication in 18 patients (82%) was inflammatory myositis (i.e., dermatomyositis, antisynthetase syndrome, overlap and necrotizing myositis); the remaining indications were SLE (n=2), Sjogren’s syndrome (n=1), and cutaneous vasculitis (n=1). Patients had a mean of 15 infusions (SD 14) spanning 1271 total hours.
Of 21 patients with at least 3 months of follow-up after IVIg initiation, 18 (86%) showed clinical improvement. Of these 18 ‘responders’, 14 had clinical follow-up at 3 months following cessation of therapy and of these, 10 (71%) had stable or quiescent disease while 4 (29%) had relapsed.
We identified 11 potential AEs in 7 patients, representing 3.2 events per 100 IVIg infusions (95% CI 1.8-5.8). AEs included headache (6), urticaria (3, one with angioedema), chills with back pain (1), and hypertension (1); none of these required an emergency room visit or hospitalization. One patient was switched from IV to subcutaneous Ig. The appropriate IVIg dose could be calculated for 15 patients (height not recorded in the remainder). We found that 7 (47%) received > 100 g excess IVIg over their treatment period and the total cumulative excess was 1242g. The cost of IVIg ranged from 61-90$/g, giving a total estimated procurement cost of $1.48 million during the study period.
Conclusion: The majority of our rheumatic disease patients received IVIg for inflammatory myositis. Most patients (86%) improved 3 months into therapy and a significant proportion of those (29%) relapsed after stopping therapy. Nearly 1 in 3 patients had a potential IVIg-related AE. A treatment course cost up to 254,964$, and one potential area of improvement is using recommended ideal body weight-based dosing.
To cite this abstract in AMA style:
Lambert-Fliszar F, Vinet E, Bernatsky S, Mendel A. Use, Procurement Cost, and Adverse Events from IVIg Use in Rheumatic Disease [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/use-procurement-cost-and-adverse-events-from-ivig-use-in-rheumatic-disease/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/use-procurement-cost-and-adverse-events-from-ivig-use-in-rheumatic-disease/