Session Information
Date: Tuesday, October 28, 2025
Title: (2437–2469) Systemic Lupus Erythematosus – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Lupus nephritis (LN) is common in pediatric patients with systemic lupus erythematosus (SLE), with more than 50% developing renal manifestations, typically within the first 2 years. Treatment options for this patient population have been inadequately studied.1 Voclosporin is approved for use in adult patients with active LN. The Phase 3 AURORA studies showed that in patients ≥18 years, adding voclosporin to MMF and low-dose glucocorticoids yielded significant reductions in proteinuria with no unexpected safety signals associated with up to 3 years of treatment.2,3 The safety and efficacy of voclosporin in patients < 18 years old has not been established. A study in the pediatric population is ongoing (Voclosporin in Adolescents with Lupus Nephritis [VOCAL; NCT05288855]). There have been reports of off-label voclosporin use in patients < 18 years captured in Aurinia’s global safety database as part of post-marketing spontaneous safety surveillance. Our objective was to summarize the post-marketing experience with voclosporin in the pediatric population.
Methods: Data included in this analysis were collected up until January 21, 2025, from the Aurinia Global Safety Database, which contains spontaneous, post-marketing reports of pediatric use. Post-marketing safety data are collected via a passive surveillance system with limitations, including possible underreporting, biased or incomplete reporting, and difficulties in attributing an adverse event (AE) to any specific drug. Use of voclosporin in patients < 18 years is considered an off-label use and is tracked in the safety database even in the absence of associated AEs. Data from the ongoing VOCAL study are not included in this analysis.
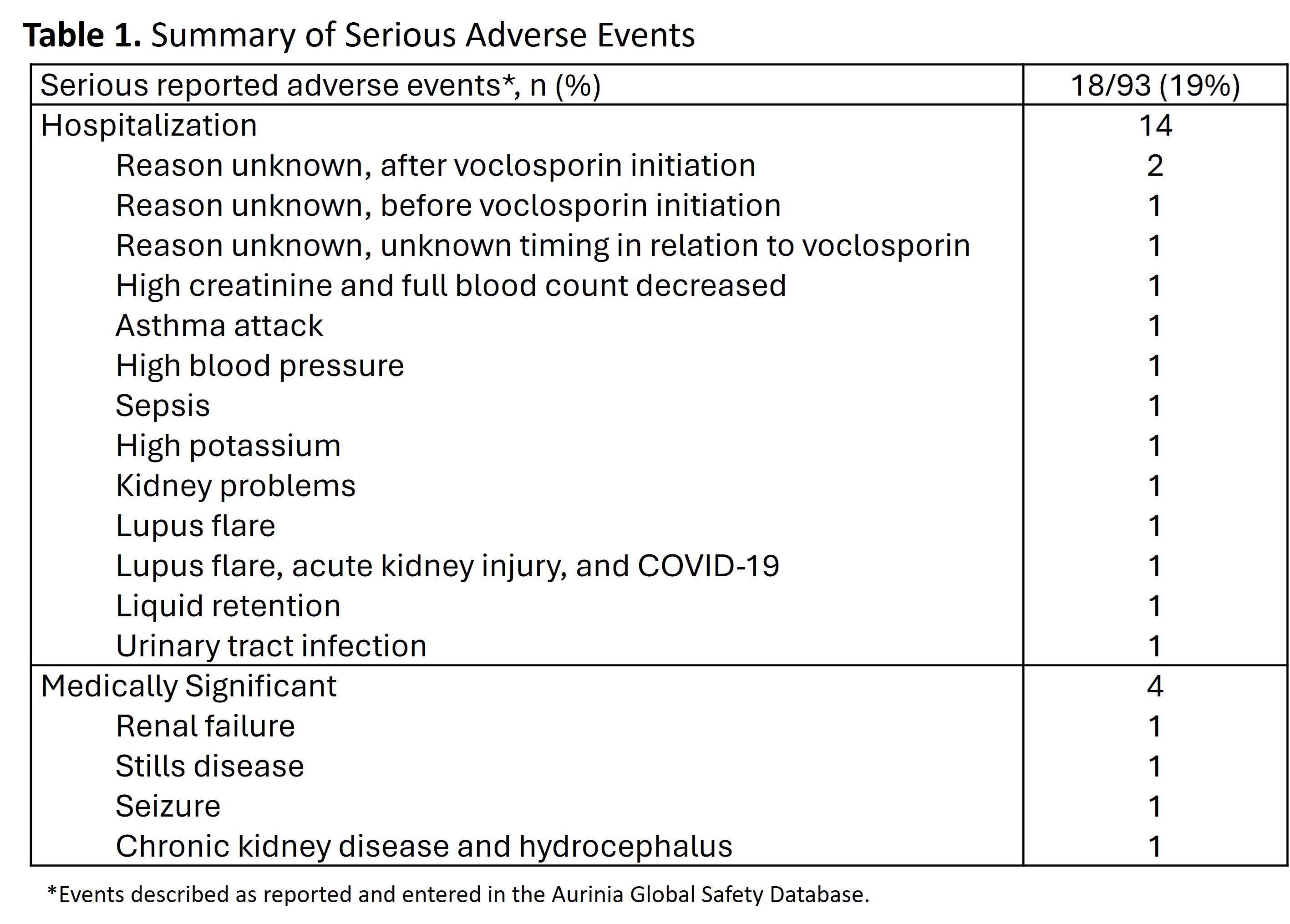
Results: As of January 21, 2025, 93 reports of pediatric use of voclosporin (in 70 unique patients) had been received, the majority from the US. Patients ranged in age from 7 to 17 years old and were mostly female. The most common indication for voclosporin was LN (83.8%). In most cases, the dose of voclosporin administered was the approved adult dose (23.7 mg twice daily; 57/93 reports). The duration of use of voclosporin was not consistently reported; where reported, the duration ranged from several weeks to 1 year, 9 months. In total, 44 of the 93 (47%) individual reports of pediatric use did not include any associated AEs following the initiation of voclosporin other than off-label use and other non-health-related events (ie. therapy interrupted, missed dose, insurance issue). Of the individual reports of pediatric use with associated AEs, 18 were considered to be serious (Table 1). There were no particular trends in the AEs reported. Data on kidney biopsies and concomitant medications were not consistently available.
Conclusion: Due to the limited number of reports and incomplete nature of spontaneous reporting, the emerging safety profile of voclosporin in pediatric patients requires further investigation, although no new safety signals were detected. Additional research is needed to better characterize the use of voclosporin in this population.1. Wenderfer SE, et al. Pediatr Clin North Am. 2019;66(1):87-99.2. Rovin BH, et al. The Lancet. 2021;397(10289):2070-2080.3. Saxena A, et al. Arthitis Rheum. 2024;76(1)59-67.
To cite this abstract in AMA style:
Chock E, Daga A, Begum T, Dao K, Dardeno M. Use of Voclosporin in Pediatric Patients: A Summary of Available Data from Post-marketing Reports [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/use-of-voclosporin-in-pediatric-patients-a-summary-of-available-data-from-post-marketing-reports/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/use-of-voclosporin-in-pediatric-patients-a-summary-of-available-data-from-post-marketing-reports/