Session Information
Date: Sunday, November 8, 2020
Title: RA – Treatments Poster III: PROs, Biomarkers, Systemic Inflammation & Radiographs
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: The multi-biomarker disease activity (MBDA; Vectra® DA, Myriad Genetics, Inc.) score is calculated from concentrations of 12 serum proteins to assess disease activity (DA) of patients with RA (Centola et al. PLoS One 2013;8(4):e60635). In a phase 3 randomized controlled trial (RCT), efficacy of the infliximab (IFX) biosimilar IFX-qbtx (PF‑06438179/GP1111) was equivalent to that of reference IFX sourced from the European Union (IFX-EU; Janssen Biologics B.V., Leiden, The Netherlands) in patients with active, moderate to severe RA (Cohen et al. Arthritis Res Ther 2018;20(1):155). In this exploratory analysis, we compared MBDA scores between treatment groups in this RCT to determine the utility of this approach as an assessment of biosimilarity.
Methods: All 650 patients in the study met the 2010 ACR/EULAR classification criteria for RA and had disease duration of ≥4 months. Patients were randomized 1:1 to IFX-qbtx or IFX-EU (3 mg/kg intravenous at weeks 0, 2, 6, and then every 8 weeks), both given with MTX (10-25 mg/week). Mean values of MBDA scores (low: 1 to < 30; moderate: 30 to ≤44; high: >44–100) were calculated at baseline (BL), and at weeks 6, 14, 30, 54, and 78 by combining the concentrations of 12 serum biomarkers using the Vectra® DA algorithm. Mean MBDA scores were compared between treatment groups. Results are reported from treatment period 1 (weeks 0–30). Analyses were performed using the intent-to-treat (ITT) population, without imputation for missing data, and data were summarized using descriptive statistics.
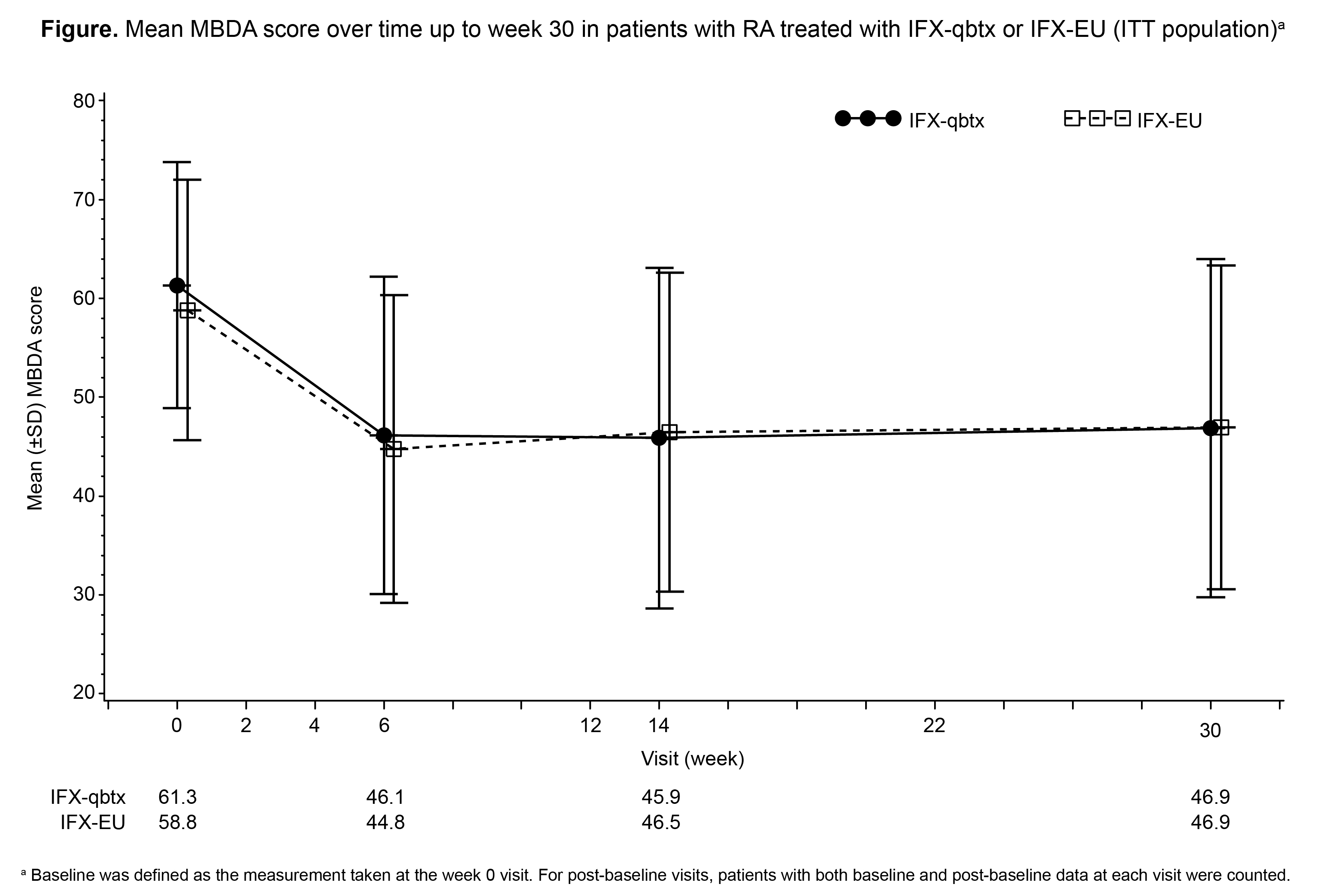
Results: At baseline (BL), mean (± standard deviation [SD]) MBDA scores for IFX-qbtx (n=236) and IFX-EU (n=248) were 61.3 (±12.5) and 58.8 (13.2), respectively. Mean MBDA scores were comparable between IFX-qbtx and IFX-EU at all measured time points through week 30 (Figure). Similar proportions in the IFX-qbtx (66.0%) and IFX-EU (66.9%) groups had high ( >44) MBDA scores at BL; the proportions of patients with high MBDA scores decreased to 42.3% and 40.2%, respectively, at week 30. Mean MBDA scores decreased by 15.2 and 14.1 from BL to week 6 in the IFX-qbtx and IFX-EU groups, respectively, and remained stable between weeks 6 and 30. Changes in the concentrations of individual biomarkers over time were generally similar between treatment groups. Changes from BL in MBDA scores correlated positively with changes from BL in high-sensitivity CRP (hs-CRP) results (Table).
Conclusion: This RCT of IFX-qbtx and IFX-EU comparing efficacy of a biosimilar and its reference product is the first study that incorporated MBDA score as an assessment of biosimilarity. Changes in MBDA scores over time were similar between IFX-qbtx and IFX-EU groups. Use of an MBDA score based on serum biomarker levels provides a sensitive assessment of biosimilarity that is independent of DA measures, which require physical examination of the patient or subjective patient global assessment.
To cite this abstract in AMA style:
Kay J, Alvarez D, Rehman M, Zhang M, Iikuni N. Use of Multi-Biomarker Disease Activity Scores to Assess Biosimilarity in a Phase 3 Randomized Controlled Trial Comparing Biosimilar Infliximab-qbtx (PF‑06438179/GP1111) with EU-Sourced Reference Infliximab in Patients with Active RA [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/use-of-multi-biomarker-disease-activity-scores-to-assess-biosimilarity-in-a-phase-3-randomized-controlled-trial-comparing-biosimilar-infliximab-qbtx-pf%e2%80%9106438179-gp1111-with-eu-sourced-refere/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/use-of-multi-biomarker-disease-activity-scores-to-assess-biosimilarity-in-a-phase-3-randomized-controlled-trial-comparing-biosimilar-infliximab-qbtx-pf%e2%80%9106438179-gp1111-with-eu-sourced-refere/