Session Information
Date: Sunday, November 13, 2022
Title: Systemic Sclerosis and Related Disorders – Clinical Poster II
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Some gastrointestinal (GI) and nutritional factors are associated with the presence of interstitial lung disease related to systemic sclerosis (SSc-ILD). However, there are many unexplored GI risk factors for the presence of SSc-ILD which could be potentially revealed by machine learning algorithms (ML). Therefore, the aim of our study was to identify GI related risk factors for the presence of SSc-ILD using ML based on decision trees (DT).
Methods: Data of the last follow-up visit from consecutive patients fulfilling the 2013 ACR/EULAR SSc classification criteria recorded in our local EUSTAR registry was analyzed.
The study outcome was the presence of SSc-ILD on high-resolution computed tomography.
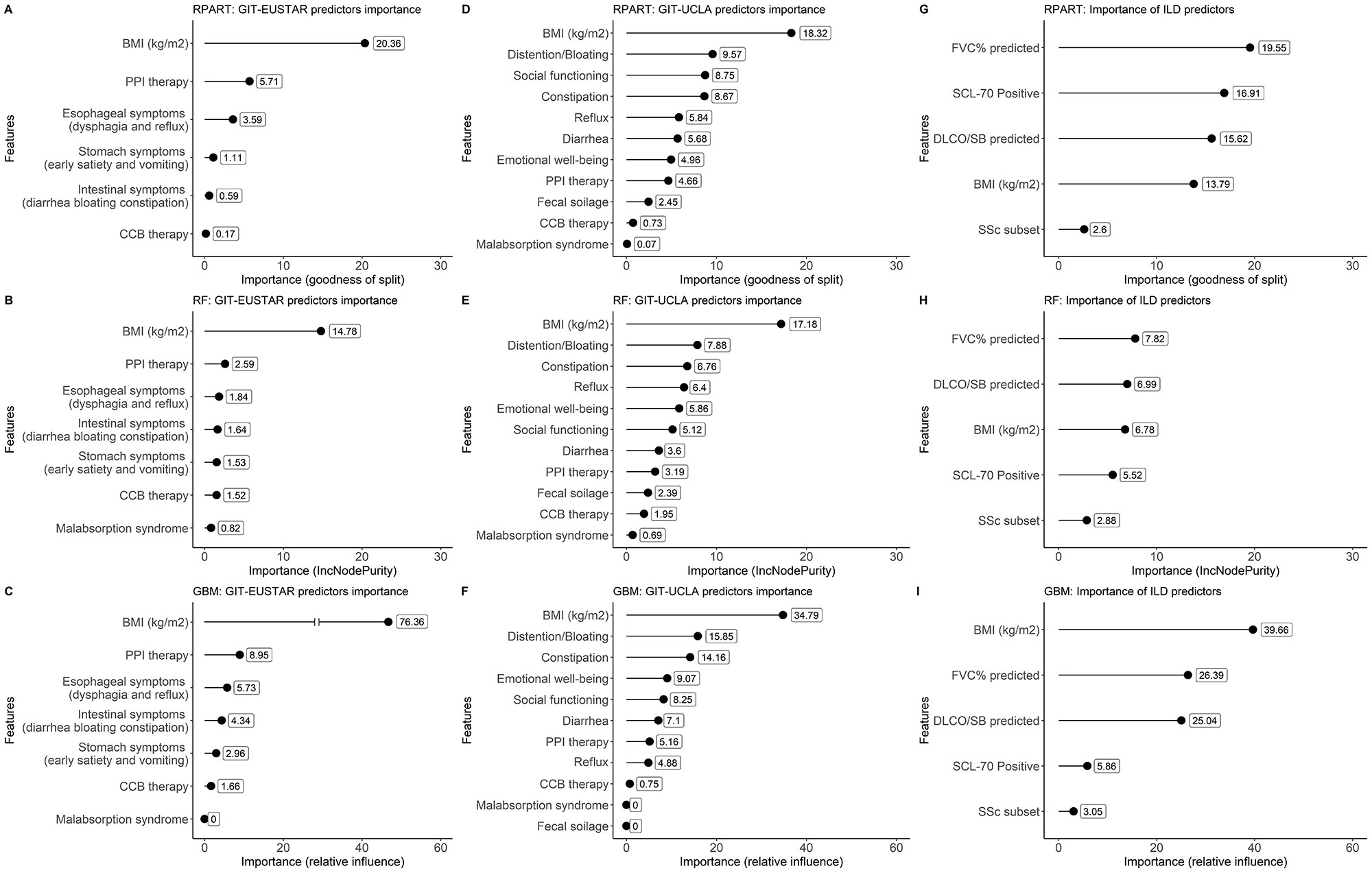
Two sets of predictors were identified based on their potential association with GI. The first set contained the following variables available in the EUSTAR registry: esophageal symptoms (dysphagia and reflux), stomach symptoms (early satiety, vomiting), intestinal symptoms (diarrhea, bloating and constipation), malabsorption syndrome, body mass index (BMI) and proton pump inhibitor (PPI) therapy and calcium channel blocker (CCB) therapy. In the second set, we replaced the first three EUSTAR variables of the first set with the scales of the UCLA Gastrointestinal Tract Questionnaire 2.0 (UCLA-GIT). Of these two sets, the most important variable was selected using three different DT-based algorithms: recursive partitioning and regression trees (RPART), random forest (RF), and gradient boosting machines (GBM).
The selected variables were eventually integrated with established predictors for presence of SSc-ILD (diffuse cutaneous subset, anti-Scl-70 positivity, male sex, forced vital capacity [FVC%] and diffusion capacity of the lung for carbon monoxide-single breath [DLCO-SB%]) into final prediction models for SSc-ILD using RPART, RF and GBM respectively. Their performance was evaluated by C-statistics. The importance of the newly detected predictor was assessed by variable importance plots (VIPs).
Results: We included in our study 334 patients. The median age was 61 [IQR: 50-69] years, 59 (17.7%) were males and 266 (79.6%) had limited cutaneous SSc. Median BMI was 23 [IQR: 21-26] kg/m2, 133 (39.8%) of the patients had SSc-ILD, median FVC% 93 [IQR: 81-105] and DLCO-SB% 72.5 [56-84]. Of the UCLA-GIT scales the highest score was for the distension/bloating with a value of 0.50 [IQR: 0-1.24]. Regarding medications, 167 (50%) patients were exposed to PPI and 39 (11.7%) to CCB.
The BMI was deemed by all three algorithms as the most important predictor of SSc-ILD among both sets of GI related variables (Fig. 1A-F). The final model, which included established risk factors for presence of ILD and the BMI, supported the importance of BMI in predicting the SSc-ILD (Fig 1 G-I). The VIPs obtained by GBM ranked the BMI as the most important predictor. A lower BMI was associated with presence of SSc-ILD (C-statistics for the RPART, RF and GBM models were 0.79, 0.70 and 0.76, respectively, corresponding to a fair accuracy).
Conclusion: A lower body mass index is a novel promising factor signalizing the presence of SSc-ILD and might helpful for enrichment for future clinical trials design.
To cite this abstract in AMA style:
Garaiman A, Mihai C, Dobrota R, bruni c, Elhai M, Jordan S, Stamm L, Hoffmann-vold A, Distler O, Becker M. Use of Body Mass Index in Prediction of Interstitial Lung Disease Related to Systemic Sclerosis Revealed by Decision Trees [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/use-of-body-mass-index-in-prediction-of-interstitial-lung-disease-related-to-systemic-sclerosis-revealed-by-decision-trees/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/use-of-body-mass-index-in-prediction-of-interstitial-lung-disease-related-to-systemic-sclerosis-revealed-by-decision-trees/