Session Information
Date: Tuesday, November 14, 2023
Title: (1913–1944) Miscellaneous Rheumatic & Inflammatory Diseases Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Sarcoidosis is a chronic, multisystem, granulomatous disease commonly affecting the lungs. Immunosuppressants, particularly corticosteroids, are the mainstay of treatment. Symptoms, severity and response to treatment can follow a heterogenous pattern, presenting a clinical challenge. Positron Emission Tomography (PET) imaging with the use of Fludeoxyglucose F18 (18F-FDG) has been recommended by the American Thoracic Society guidelines in choosing an appropriate biopsy site.Use of 18F-FDG PET in disease monitoring remains uncertain. We undertook a systematic literature review (SLR) on the use of 18F-FDG PET in assessing response to treatment in adults with pulmonary sarcoidosis.
Methods: The protocol was registered on Prospero (CRD42023416412). All published articles discussing PET CT use in response to treatment in pulmonary sarcoidosis were included, until March 2023, in Medline, Embase, and Cochrane Databases. The search was restricted to English-language articles. All article types were eligible except opinion pieces, case reports, case series of ≤10 patients and reviews. Articles meeting inclusion criteria were examined by one author, with 20% validity screening. In addition to basic demographics, information was extracted on: Siltzbach classification of subjects; treatment; additional tests performed; time between baseline and follow-up PET CT.
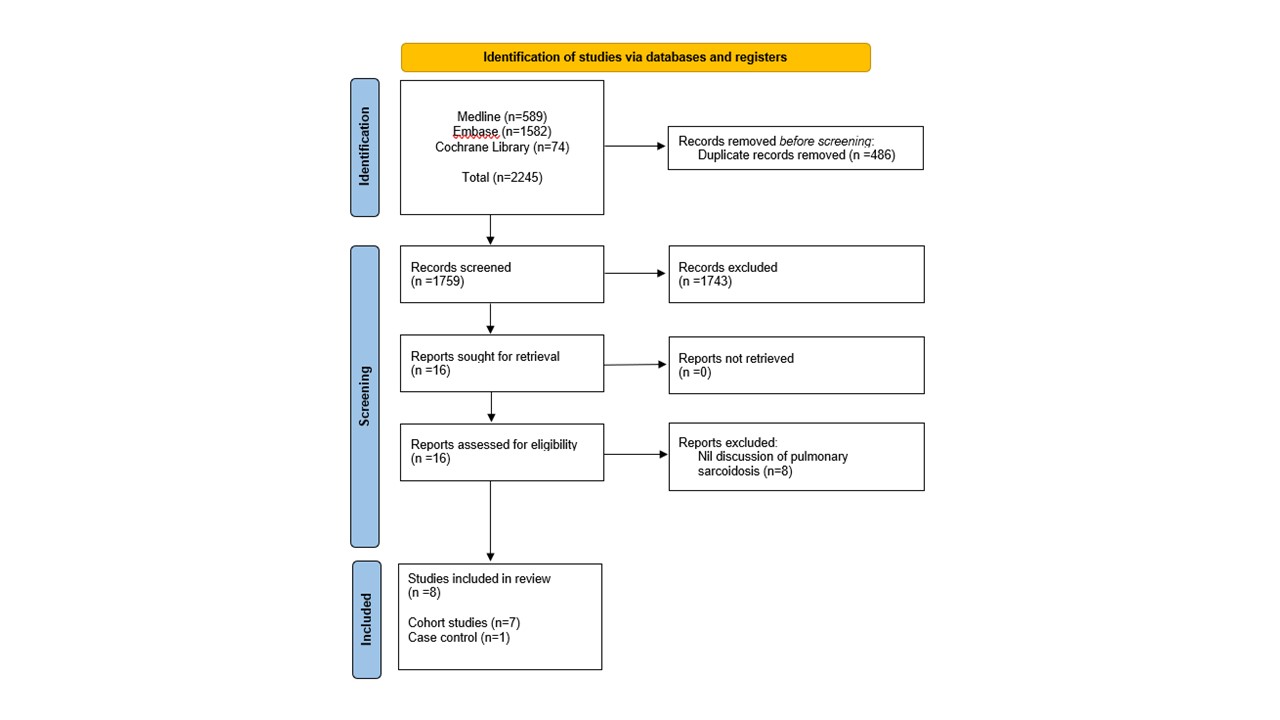
Results: Initially, 1759 articles were retrieved with 8 ultimately included (4 prospective; 3 retrospective; 1 case-control). A pooled total of 260 patients with pulmonary sarcoidosis were included, 40.7% male, mean age 47.0 years (SD 3.4). Study populations were from France (n=1), China (n=1), The Netherlands (n=1), Turkey (n=1), India (n=2) and Serbia (n=2). Treatment for pulmonary sarcoidosis varied markedly amongst the included studies, including: infliximab (n=1) and systemic corticosteroids (n=3); treatment was unknown in 4 studies. All studies used 18F-FDG-PET, except one in which gallium-67 scintigraphy was also used. Time between baseline PET CT and follow-up scan ranged from 2.8 weeks to 12 months. Compared to clinical response, sensitivity of PET CT in determining response to treatment ranged from 56% to 100%, with mean sensitivity of 75.3% (SD 16.0). Additional tests performed across all studies included spirometry, chest radiograph, serum angiotensin-converting-enzyme levels, soluble interleukin-2 receptor levels. All studies concluded that PET CT correlates with clinical response to treatment and is useful for prognostication, aside from one study which concluded that metabolic response on PET CT can predict future risk of relapses but does not correlate with clinical response.
Conclusion: To our knowledge, this is the first SLR summarising the use of 18F-FDG PET in assessing response to treatment in adults with pulmonary sarcoidosis. 18F-FDG-PET is useful in determining response to treatment and prognosis in pulmonary sarcoidosis, and may have a role in predicting future response. Further work in greater patient numbers is required to confirm the utility of PET CT in the management of pulmonary sarcoidosis.
Flow diagram of stages of systematic literature review.
Cochrane Library encompasses library of: systematic reviews; systematic review protocols; controlled clinical trials.
To cite this abstract in AMA style:
Kouranloo K, Krishnakumar M, Dey M. Use of 18F-FDG PET in Assessing Response to Treatment in Adults with Pulmonary Sarcoidosis: A Systematic Literature Review [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/use-of-18f-fdg-pet-in-assessing-response-to-treatment-in-adults-with-pulmonary-sarcoidosis-a-systematic-literature-review/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/use-of-18f-fdg-pet-in-assessing-response-to-treatment-in-adults-with-pulmonary-sarcoidosis-a-systematic-literature-review/