Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Early consensus statements recommended discontinuing tumour necrosis factor inhibitors (TNFi) during pregnancy. Despite new guidelines recommending against this, the choice to stop TNFi pre-conception is patient- and provider-dependent. Understanding TNFi discontinuation pre-conception may help inform initiatives to optimize outcomes. We examined calendar trends in TNFi discontinuation pre-conception in women with chronic inflammatory diseases and compared characteristics of those who stopped using TNFi pre-conception (without resuming in pregnancy) compared with those who used TNFi at any time during pregnancy.
Methods: We created a cohort of pregnant women with rheumatoid arthritis, ankylosing spondylitis, psoriasis/psoriatic arthritis, and/or inflammatory bowel disease who delivered between 2011 and 2019 using the MarketScan commercial database. TNFi use was defined as ≥1 filled prescription or infusion procedure claim, categorized as a) TNFi pre-conception only (i.e. ≥1 prescription filled or infusion procedure claim in the 12 weeks preceding the gestational period but not within the gestational period) or b) TNFi use at any time during pregnancy (i.e. any prescription filled or infusion procedure claim during the gestational period, including restarts, new starts, and those continuing from pre-conception).
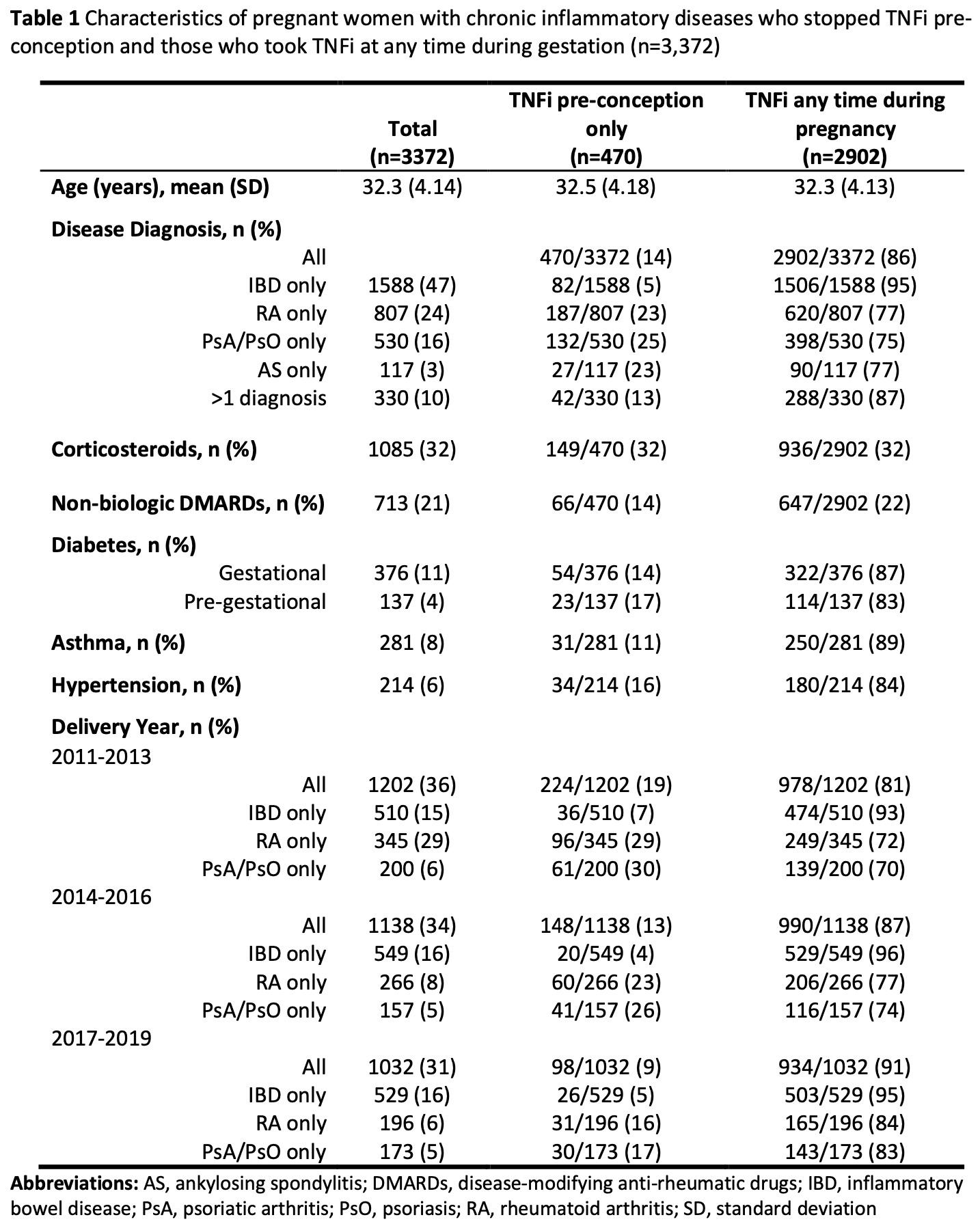
Results: We identified 3,372 pregnancies; 14% discontinued TNFi in the 12 weeks before conception and did not restart, and 86% were exposed to TNFi during pregnancy. IBD patients accounted for 47% of all pregnancies. Comparing rheumatologic to non-rheumatologic patients, more RA individuals (difference of 18%, 95% confidence interval, CI, 15-21%) and PsA/PsO (20%, 95% CI 16-24%) discontinued their TNFi than IBD patients. Corticosteroid use was similar in both TNFi exposure groups, and those using TNFi during pregnancy were more likely to use non-biologic disease-modifying agents concomitantly (difference of 8%, 95% CI 5-12%). Across comorbidities (diabetes, asthma, and hypertension), there was no difference in discontinuation. Over time, a lower proportion of patients stopped TNFi pre-conception (2011-2013 19% vs 2014-2016 13% vs 2017-2019 10%; p-value for trend < 0.0001).
Conclusion: In our study, 14% discontinued TNFi in the 12 weeks before conception and did not restart. The proportion of patients stopping TNFi pre-conception decreased over time, possibly reflecting how changes in the observational literature pre-dated (and influenced) guidelines. Further research on TNFi discontinuation in the years after the 2020 ACR guidelines is warranted to establish guideline compliance and monitor perinatal outcomes.
To cite this abstract in AMA style:
Flatman L, Bernatsky S, Malhamé I, St. Pierre Y, Basso O, Bérard A, Vinet E. Use and Discontinuation of Tumour Necrosis Factor Inhibitors Among Pregnant Women with Chronic Inflammatory Diseases [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/use-and-discontinuation-of-tumour-necrosis-factor-inhibitors-among-pregnant-women-with-chronic-inflammatory-diseases/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/use-and-discontinuation-of-tumour-necrosis-factor-inhibitors-among-pregnant-women-with-chronic-inflammatory-diseases/