Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Monocytes/macrophages are the most abundant cells infiltrating the glomeruli and in the active urinary sediment of patients with Lupus Nephritis (LN).1 These cells also have relative abundance of Toll Like Receptor 4 (TLR4) as compared to other white blood cells.2 Therefore MRP8/14 (Calprotectin), an endogenous TLR4 ligand, could be responsible for their activation in LN and may serve as a biomarker of renal disease activity in urine.
Methods: SLE patients with active nephritis (AN), active disease without nephritis (active non-renal; ANR) and inactive disease (ID) were enrolled. Disease activity was assessed using SLEDAI and renal SLEDAI (rSLEDAI). Patients in AN group were treated according to the ACR 2012 guidelines and followed up every 3 months for 1 year. AN disease was defined as SLEDAI ≥5 with rSLEDAI ≥4, ANR was defined as SLEDAI ≥5 with rSLEDAI =0 and ID was defined as SLEDAI ≤4 with rSLEDAI =0. Urine and serum samples were collected at baseline for all patients and at every 3 months for 1 year in AN group. Urine samples from 10 healthy subjects (HC) and 10 patients of rheumatoid arthritis (RA) served as controls. Serum MRP8/14 (sMRP8/14) and urinary MRP8/14 (uMRP8/14) were measured using commercially available ELISA kits (BioLegend, San Diego, CA, USA) and urinary values were normalized for creatinine excretion. Variables are expressed as median (range) and non-parametric tests were used for analysis. A p-value < 0.05 was considered significant.
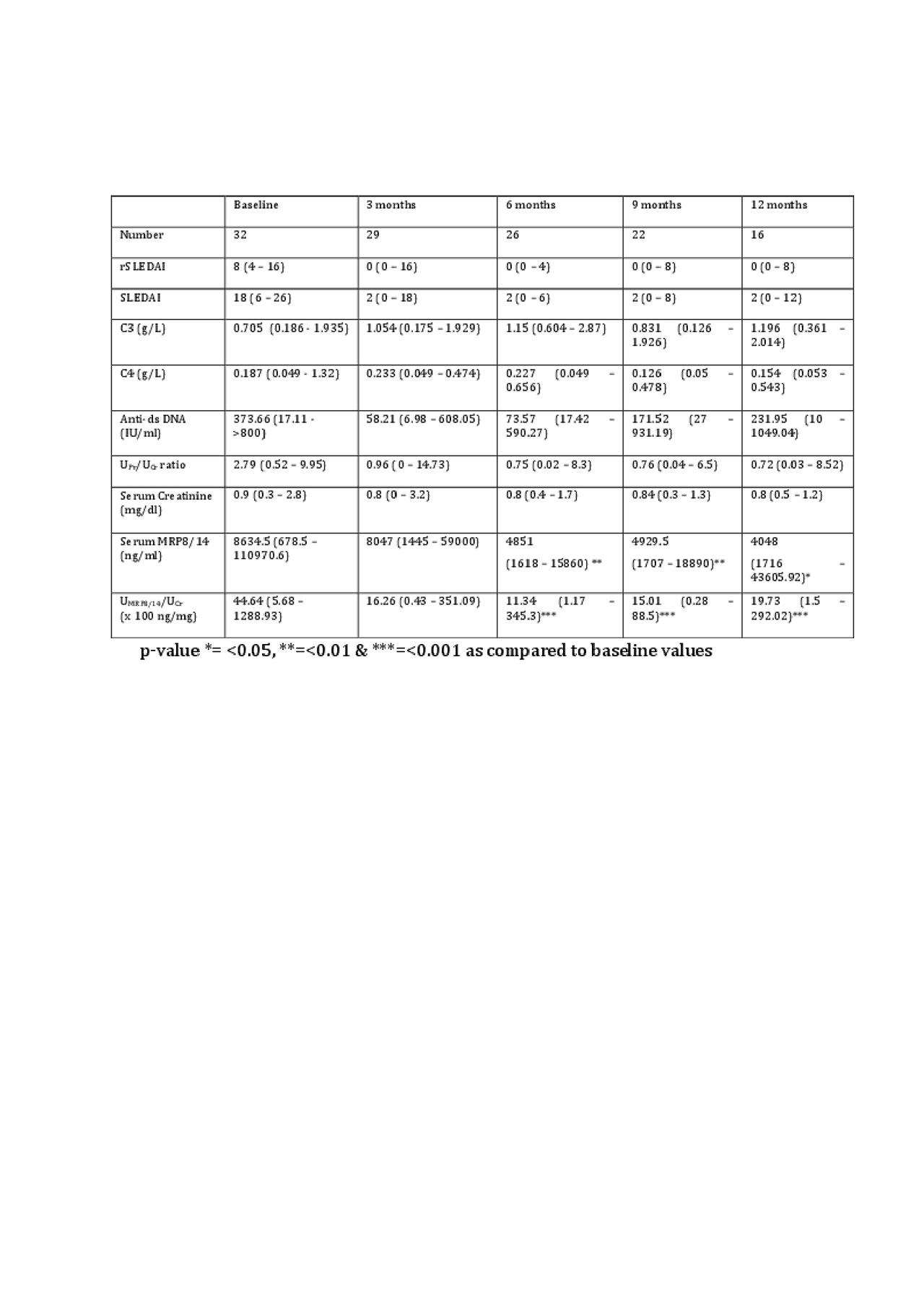
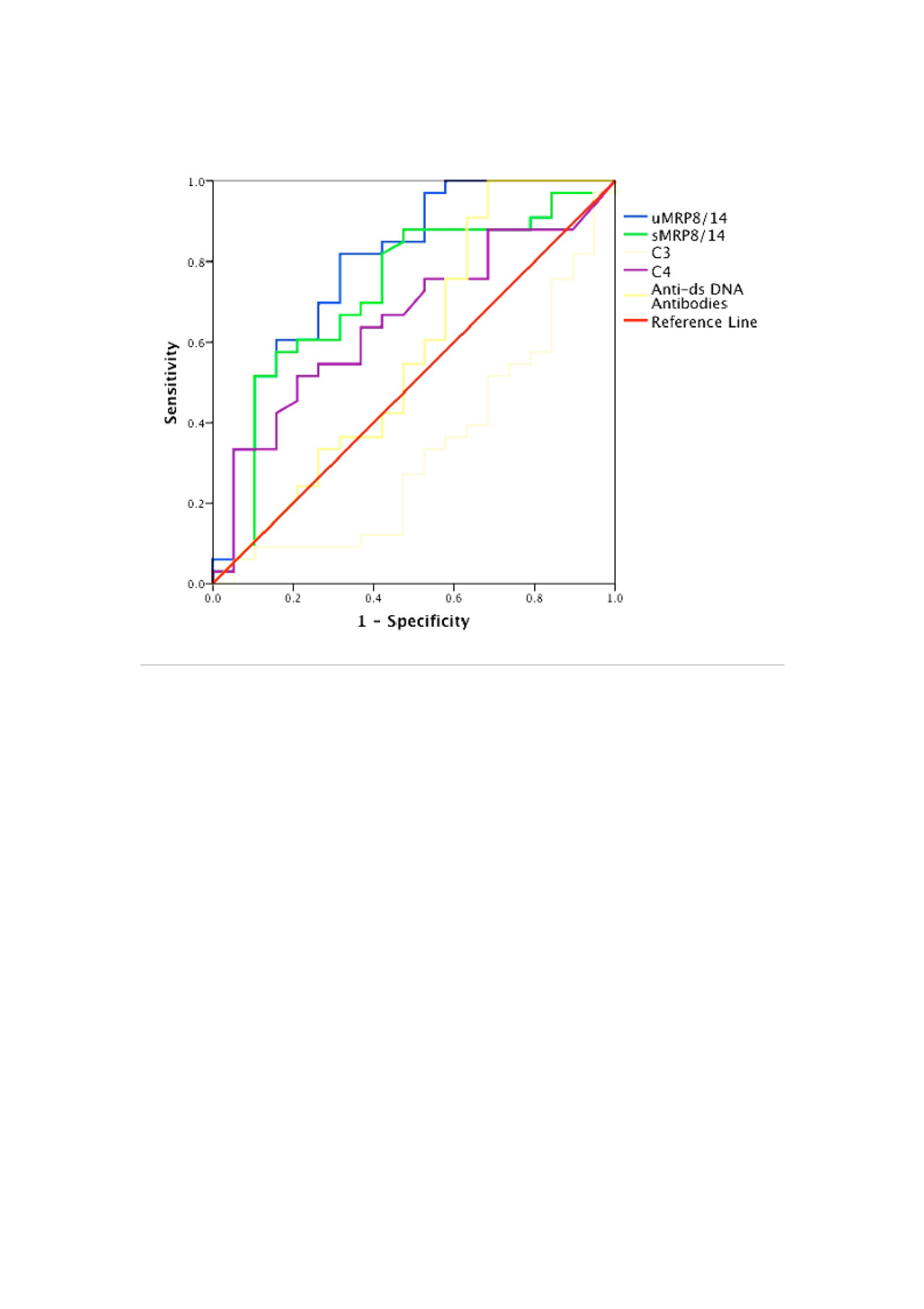
Results: A total of 52 SLE patients (F:M = 49:3) were enrolled. Among these, 32 were in AN category whereas 10 patients each were enrolled in ANR and ID categories. Complete response rates at 3, 6, 9 and 12 months were 24%, 42%, 50% and 50% respectively and 3 patients expired at 3 months because of high disease activity. At baseline, normalized uMRP8/14 (ng/mg of creatinine) was significantly higher in AN group [4464 (569-128894)] as compared to ANR [1167 (4-18357)], ID [438 (147-4320)], HC [1348 (3-2726)] and RA [339 (6-11519)] (p-value < 0.05) groups. At baseline, uMRP8/14 showed good correlation with urinary protein:creatinine ratio (r=0.55; p-value< 0.001), rSLEDAI (r=0.5, p-value < 0.001) and SLEDAI (r=0.3, p-value < 0.001) but not with sMRP8/14 (r=0.25). Baseline uMRP8/14 but not sMRP8/14 could differentiate between AN and ANR groups (Table 1) and on ROC analysis, uMRP8/14 (AUC=0.8) performed better than sMRP8/14 (AUC=0.72), C3 (AUC=0.67), C4 (AUC=0.35) and anti-ds DNA antibodies (AUC=0.58) (Figure 1). On follow-up of AN patients, with a reduction in disease activity, uMRP8/14 also decreased significantly at 6,9 and 12 months visits as compared to baseline (p-value < 0.05) (Table 2). sMRP8/14 also decreased significantly at 6 and 9 months but had an erratic and irregular trend. uMRP8/14 also showed a rise before conventional markers in 6 patients who relapsed within 1 year of follow-up requiring change of therapy.
Conclusion: uMRP8/14 is a potential biomarker of LN disease activity. In patients with active SLE, it helps differentiate between patients with and without renal involvement. It correlates with renal disease activity and has a potential to predict relapse of LN.
References:
- Arthritis Res Ther. 2015 Apr 3;17:94
- Front Immunol. 2014 Jul 10;5:316
To cite this abstract in AMA style:
Gupta R, Mitra D, Rani S. Urinary MRP8/14, an Endogenous Toll-Like Receptor 4 Ligand, Reflects Renal Disease Activity in Lupus Nephritis: A Cross-Sectional and Longitudinal Assessment [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/urinary-mrp8-14-an-endogenous-toll-like-receptor-4-ligand-reflects-renal-disease-activity-in-lupus-nephritis-a-cross-sectional-and-longitudinal-assessment/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/urinary-mrp8-14-an-endogenous-toll-like-receptor-4-ligand-reflects-renal-disease-activity-in-lupus-nephritis-a-cross-sectional-and-longitudinal-assessment/