Session Information
Date: Wednesday, November 15, 2023
Title: Abstracts: SLE – Diagnosis, Manifestations, & Outcomes IV: Outcomes & Comorbidity
Session Type: Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Many studies of cancer risk in SLE are limited by small sample size or use of administrative data, which rely on billing code diagnoses instead of clinical data. We produced updated estimates cancer risk in the largest-ever cohort of clinically confirmed incident SLE patients.
Methods: Adults with SLE were enrolled (within 15 months of diagnosis) into the SLICC Inception Cohort, across 32 centres. Individuals were followed with annual assessments, where detailed data was collected on Disease Activity Index-2000 (SLEDAI-2K), lupus drugs in the past year, and documentation of cancer diagnoses (by the examining physician at yearly intervals).
We performed univariate and multivariate Cox regression, with baseline demographics (age at SLE onset, race/ethnicity, sex), and time-dependent variables for medications (antimalarials, biologics and other immunosuppressives, corticosteroids), smoking, and SLEDAI-2K. As well as cancer over-all, we evaluated risk factors for the most common cancer types.
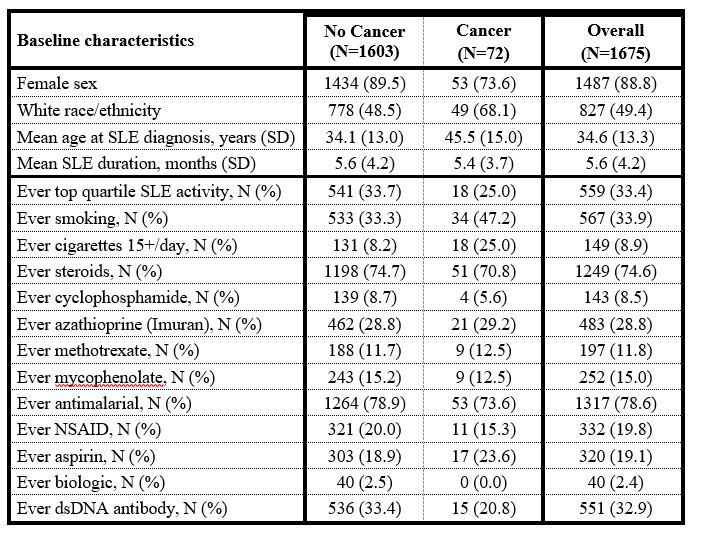
Results: Of 1848 new-onset SLE patients enrolled between 1999-2011, 1675 had at least one follow-up; these were the sample for the current analysis. End date was the first of death, last visit, or end of study interval for this analysis (June 21,2021). Baseline demographics are shown in Table 1.
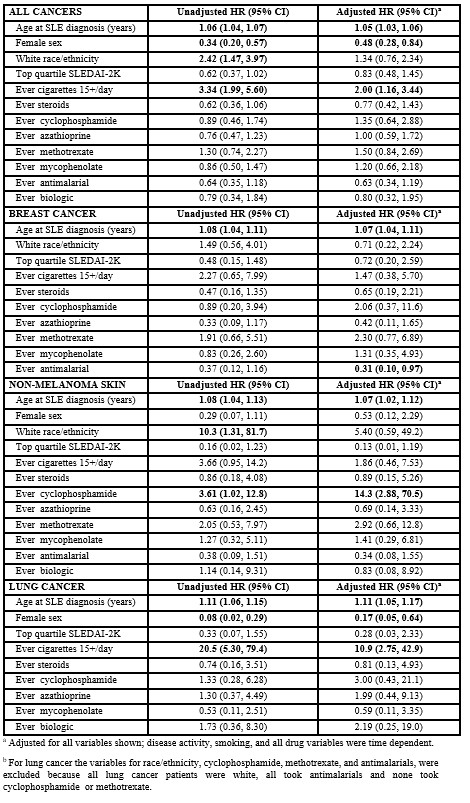
Over 16493 years of follow-up (median follow up, 10 years) 72 cancers occurred (incidence 4. 4 events per 1,000 patient-years). This included 16 breast cancers, 10 non-melanoma skin, 10 lung, 6 hematological, 6 prostate, 5 melanoma, 3 cervical, 3 renal, 3 head and neck, 3 thyroid, 2 gastric, 2 uterine, and one each rectal, sarcoma, thymoma. Adjusted and unadjusted hazard ratios (HR) are shown in Table 2. Multivariate analyses of all cancer types (analyzed together) suggested a higher risk of cancer among patients of older age, males, and those who smoked more than 15 cigarettes a day.
In the multivariate analyses specifically for breast cancer, age at SLE diagnoses remained a risk factor, and antimalarials were associated with a decreased risk. This effect of antimalarials was not clearly seen for any other cancer type. For non-melanoma skin cancer, both age at SLE diagnosis and cyclophosphamide (albeit with a wide confidence interval) were associated with risk. For hematologic cancers, older age was a risk factor in univariate analyses (hazard ratio, HR 1.06, 95% confidence interval, CI 1.01 , 1.12) as was highest quartile of SLEDAI-2k (HR 10.3, 95% CI 1.31 , 81.7), but due to the low number of hematologic cancers, multivariate estimates were less precise and confidence intervals included the null value for all variables.
Conclusion: Our updated analyses of this large, international inception SLE cohort continues to illustrate how different cancer types in SLE might be associated with specific risk factors. Additional follow-up is still needed to precisely determine the potential effects of disease activity (particularly for hematologic malignancies) and medications on cancer risk in SLE.
To cite this abstract in AMA style:
Bernatsky S, Ramsey-Goldman R, Urowitz M, Hanly J, Gordon C, Petri M, Ginzler E, Wallace D, Bae S, Romero-Diaz J, Dooley M, Peschken C, Isenberg D, Rahman A, Manzi S, Jacobsen S, Lim S, van Vollenhoven R, Nived O, Kamen D, Aranow C, Buyon J, Ruiz-Irastorza G, Sanchez-Guerrero F, Gladman D, Fortin P, Lee J, Lukusa L, Alarcón G, Merrill J, Kalunian K, Ramos-Casals M, Steinsson K, Zoma A, Askanase A, Khamashta M, Bruce I, Inanç M, Clarke A. Updated Analyses of Cancer Incidence and Risk Factors in a Large International SLE Cohort [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/updated-analyses-of-cancer-incidence-and-risk-factors-in-a-large-international-sle-cohort/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/updated-analyses-of-cancer-incidence-and-risk-factors-in-a-large-international-sle-cohort/