Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Poor treatment adherence and persistence is an ongoing problem among SLE patients due to complex regimens and may lead to frequent use of high-dose corticosteroids, despite their harmful side effects. Real-world data are helpful to understand adherence to SLE treatment regimens and concomitant corticosteroid (CS) utilization as newer treatments are available. We describe concomitant CS use and adherence to antimalarials, immunosuppressants, and biologics among adult patients with newly diagnosed SLE in the US.
Methods: This retrospective observational cohort study used IBM MarketScan® Commercial and Medicare Supplemental Databases to include patients aged ≥18 years, newly diagnosed with SLE, and without prior antimalarial, immunosuppressant, or biologic SLE treatment. The first SLE diagnosis date was defined as index date. Patients with an SLE diagnosis between January 2013 and December 2017, and having ≥24 months of continuous enrollment, prior to and following index date, were included in study. Concomitant CS use (administration route and dose), SLE medication adherence (defined as medication possession ratio [MPR] ≥0.8), and persistence (defined as no gap ≥60 days in treatment) were described during up to 3 lines of therapy (LOTs). Date of the earliest prescription for SLE treatment, on or following the index date, was defined as LOT1. A new medication (added or switched to) resulted in a new LOT. Up to 3 lines of therapy were considered among SLE patients newly initiating SLE treatment during follow-up.
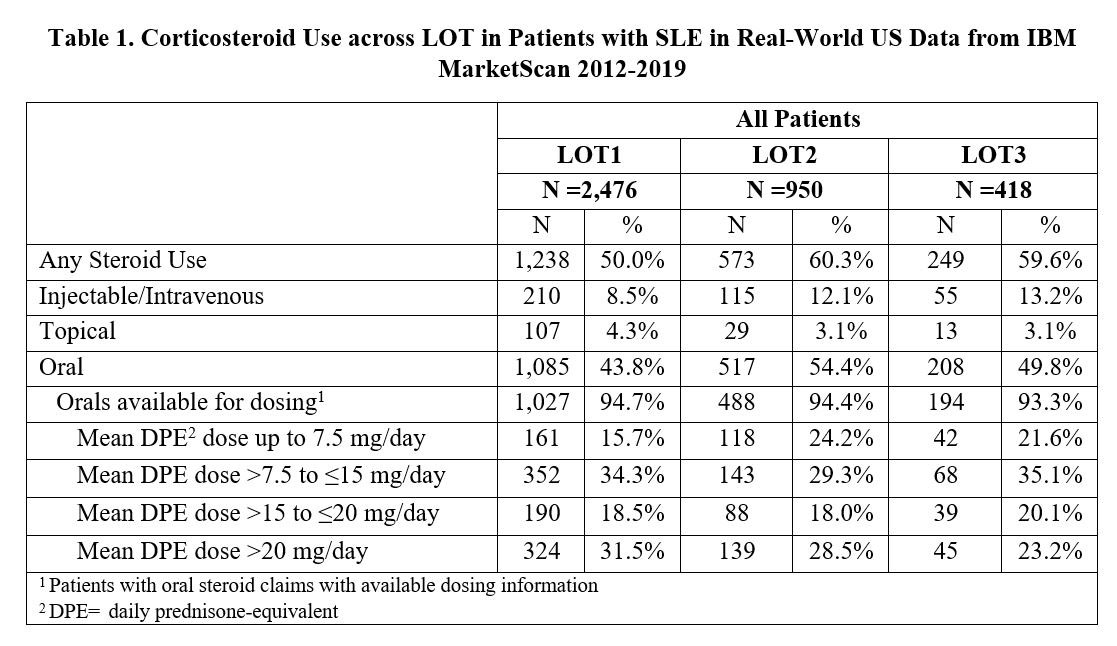
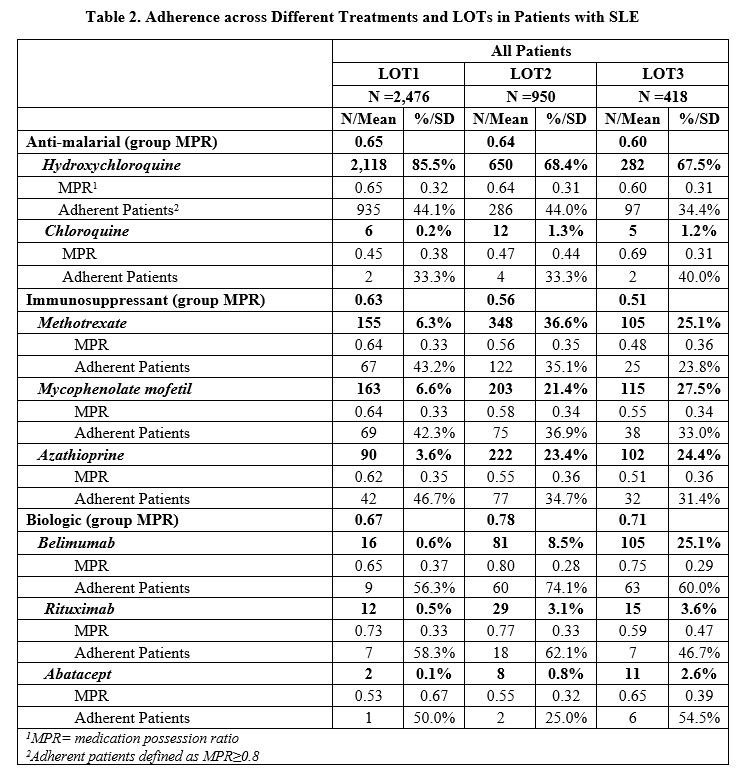
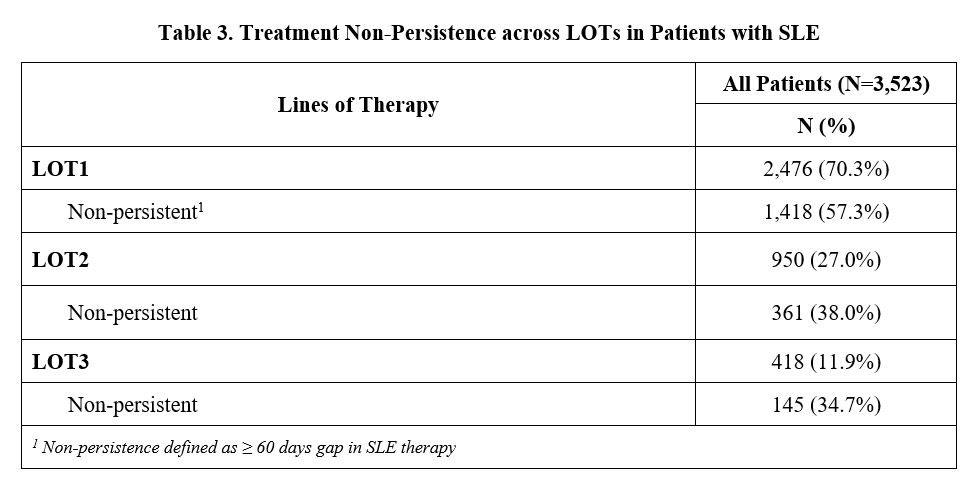
Results: A total of 2,476 (70.3%) of 3,523 newly diagnosed SLE patients initiated a LOT1, 950 (27.0%) advanced to LOT2, and 418 (11.9%) moved to LOT3 during the 2-year follow-up period. Concomitant CS use was lower in LOT1 (50%) than in LOT2 and LOT3 (60%) (Table 1). Across all LOTs, 75-84% of oral CS users had a daily prednisone-equivalent dose (DPE) greater than 7.5 mg. DPE use over 20 mg occurred in 23-31% of patients. Adherence to biologics was highest (average MPR=0.71) vs. antimalarials (0.65) and immunosuppressants (0.56), regardless of LOT, with over half of patients taking belimumab having an MPR≥0.8 (Table 2). Less than half of patients taking antimalarials or immunosuppressants were adherent with an MPR ≥0.8 across all LOTs. Non-persistence across all medications was 57.3% during LOT1, 38.0% during LOT2, and 34.7% during LOT3 (Table 3).
Conclusion: While on SLE therapy, over 75% of patients are using doses of oral CS greater than physiologic dose (≥7.5 mg/day). Adherence and persistence remained consistently low across all LOTs. While patients who advanced to a next LOT improved their persistence, they continued on high doses of concomitant CS. These findings suggest an unmet need for new SLE treatments with higher adherence and persistence, as well as the ability to reduce the use of CS.
To cite this abstract in AMA style:
Masurkar P, Reckleff J, Princic N, Limone B, Schwartz H, Karis E, Zollars E, Stolshek B, Costenbader K. Unmet Need in Systemic Lupus Erythematosus (SLE) Therapy: High Corticosteroid Use and Poor Adherence and Persistence to SLE Treatments in the US [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/unmet-need-in-systemic-lupus-erythematosus-sle-therapy-high-corticosteroid-use-and-poor-adherence-and-persistence-to-sle-treatments-in-the-us/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/unmet-need-in-systemic-lupus-erythematosus-sle-therapy-high-corticosteroid-use-and-poor-adherence-and-persistence-to-sle-treatments-in-the-us/