Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Photosensitivity is a hallmark symptom of lupus erythematosus (LE), in which patients develop inflammatory skin lesions in response to ultraviolet radiation (UVR). In examining mechanisms underlying photosensitivity, we reported that healthy Langerhans cells (LCs) limit UVR-induced keratinocyte apoptosis and skin injury via ADAM17 (a disintegrin and metalloprotease 17)-mediated epidermal growth factor receptor (EGFR) ligand activation. We also showed evidence of reduced LC ADAM17 activity in murine models and human SLE and that this LC ADAM17 dysfunction contributed to photosensitivity. Why LCs should be dysfunctional in LE is not known. As a type I interferon (IFN-I) signature is prominent in human LE non-lesional skin, and anifrolumab (anti-IFNAR) has beneficial effects on cutaneous disease in SLE, we tested the hypothesis that IFN-I contributed to LC ADAM17 dysfunction and delineated a mechanism for this IFN-I-LC ADAM17 axis.
Methods: To assess IFN-I signature, among other gene expression profiles, microarray of non-lesional, human cutaneous LE skin and RNA sequencing of whole skin from lupus mouse models were performed and characterized. To characterize human LC ADAM17 activity and expression, suction blistering was performed on healthy participants as previously described: Eight 5 mm suction blisters were generated with a negative pressure instrument on the arm of healthy donors over 30-60 minutes. The blister fluid and epidermal roofs were collected for flow cytometry-based assays. All human tissue collection and research use adhered to protocols approved by the Institutional Review and Privacy Board at the Hospital for Special Surgery (IRB# 2019-1998), where participants signed written informed consents. To quantify murine LC ADAM17 activity and expression and LC reactive oxygen species (ROS), tissue were digested, and flow cytometric-based assays were conducted. To test for photosensitivity, readouts of skin inflammation and cellular infiltrate were measured.
Results: We find IFN-I signatures in non-lesional skin of MRL/lpr and B6.Sle1yaa photosensitive lupus models in addition to that of human cutaneous lupus erythematosus (CLE) patients, suggesting that SLE model mice can be used to study a hypothesized IFN-LC ADAM17 axis. We show that IFN-I is sufficient to reduce human and murine LC ADAM17 activity independently of surface ADAM17 and that anti-IFNAR improves LC ADAM17 activity and reduces photosensitivity in EGFR and LC ADAM17-dependent manners. IFN-I reduced LC reactive oxygen species (ROS) generation that, in turn, diminished LC ADAM17 activity.
Conclusion: Together, our results establish an IFN-I-ROS-LC ADAM17 axis that contributes to photosensitivity in LE. Our results suggest that anifrolumab has ameliorative effects on SLE skin disease at least in part by restoring LC function and points to LCs as a therapeutic target in LE.
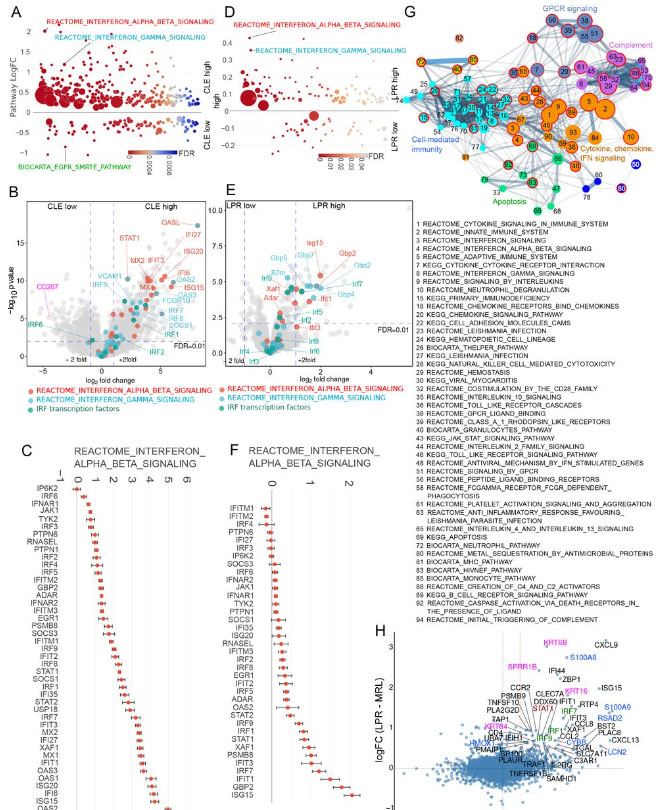
share upregulated interferon (IFN) signature. Non-lesional skin gene expression
from (A-C) human CLE patients (N=7) and healthy controls (N=13) analyzed by
microarray and (D-F) MRL/lpr (LPR) (N=8) and control MRL/+ (MRL) (N=9) mice
analyzed by RNA sequencing. (A, D) Differentially expressed pathways were
determined using QuSAGE pathway analysis against Molecular Signatures Database
(MSigDB). (B, E) Volcano plots of differentially expressed genes. Genes from IFN α/β
(red), IFNγ (blue) and IRF transcription factor (green) pathways are marked. Cd207
(pink) is additionally marked in (B). (C, F) Log-transformed expression fold change for
genes in the IFN α/β pathway. (G) Pathway similarity network built for the most
enriched pathways (adjusted p<0.005) in CLE non-lesional skin determined by ranked
GSEA (a complete list is in Table S5). Colors indicate network community clusters
identified by the walktrap algorithm. Red borders indicate pathways that were also
enriched in MRL/lpr mice (listed). (H) Comparison of gene expression changes from
CLE (from B) and MRL/lpr mice (from E).
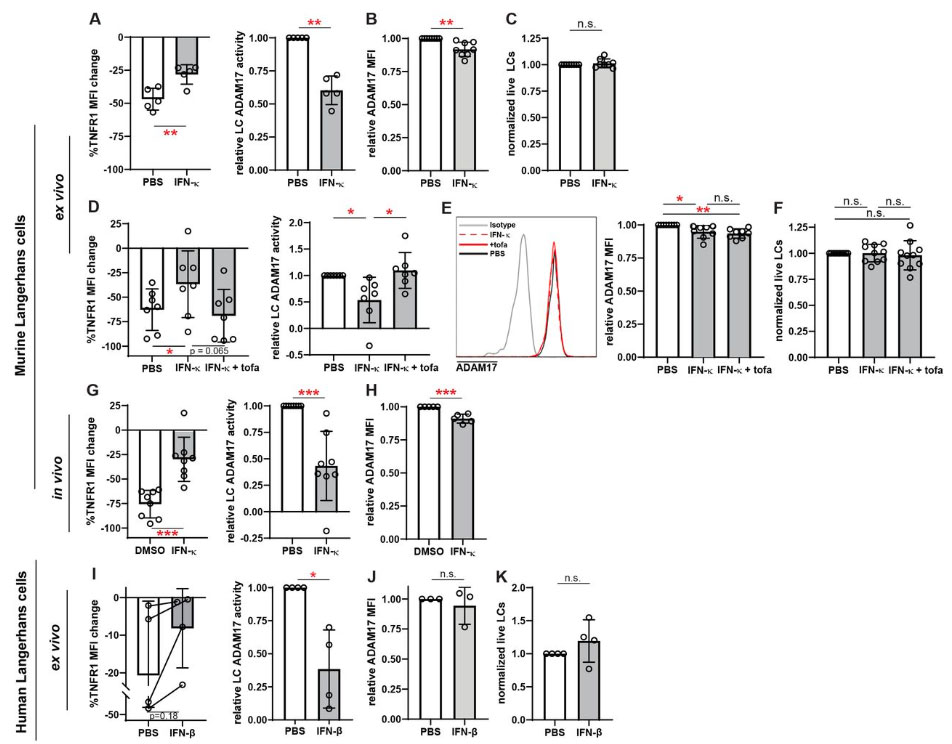
ADAM17 sheddase activity, (A-C) epidermal cell suspensions from WT mice , (D-F) WT mice, and (GH)
suction blister epidermal cell suspensions from healthy human donors were treated with IFNκ, IFNκ
+tofacitinib, or vehicle as indicated. For (D-F), treatments were applied topically to the ears for 16_20
hours. (A, D, G, I) LC ADAM17 sheddase activity as indicated by the extent of UVR-induced cell
surface TNFR1 loss. (Left) %reduction in cell surface TNFR1 mean fluorescence intensity (MFI).
(Right) LC ADAM17 activity is plotted as a function of the relative reduction in TNFR1 loss, normalized
to the change in the vehicle condition. (B, E, H, J) Cell surface ADAM17 expression levels as indicated
by MFI relative to that of MRL/+ isotype controls.(C, F, K) Relative numbers of LCs after indicated
treatments. Each symbol represents (A-F, I-K) cells from a single mouse or healthy human donor
across conditions or (G-H) a single mouse; bars represent average values and error bars are SD.
n=4_10 per condition. *p<.05, **p<.01, ***p<.001, n.s.=not significant by unpaired t-test.
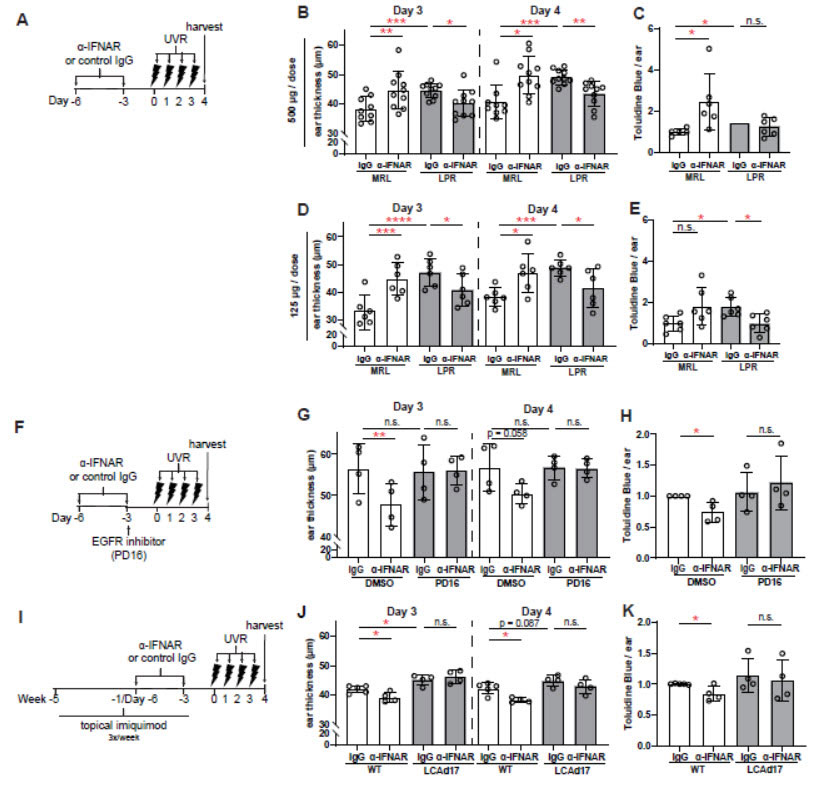
MRL/lpr and (I-K) LCAd17 mice and their controls were treated according to schematics in (A, F, I).
(B,D,G,J) Ear thickness. (C, E, H, K) Epidermal permeability as indicated by toluidine blue retention. (B-E,
G-H, J-K) Each symbol represents one mouse, bars represent average values, and error bars are SD.
n=4-6 per condition. *p<.05, **p<.01, ***p<.001, n.s.=not significant by paired (B, D, G, J) and unpaired (C,
E, H, K) t-test.
To cite this abstract in AMA style:
Li T, Seltzer E, Zyulina V, Veiga K, Schwartz N, Chinenov Y, Oliver D, Lora J, Jabbari A, Liu Y, Munir H, Shipman W, Sandoval M, Sollohub I, ambler w, Mishra B, taber s, onel k, Rashighi M, krueger j, Anandasabapathy N, Blobel C, Lu t. Understanding How Type I Interferon Modulates Langerhans Cell ADAM17 to Promote Photosensitivity in Lupus [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/understanding-how-type-i-interferon-modulates-langerhans-cell-adam17-to-promote-photosensitivity-in-lupus/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/understanding-how-type-i-interferon-modulates-langerhans-cell-adam17-to-promote-photosensitivity-in-lupus/