Session Information
Date: Sunday, November 12, 2023
Title: (0510–0542) Spondyloarthritis Including Psoriatic Arthritis – Treatment: AxSpA Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Anti-IL17 agent secukinumab (SEC) has been proven effective and is widely used in the treatment of spondyloarthritis (SpA) patients alternatively to tumor necrosis factor inhibitors (TNFis). Our aim was to compare treatment retention and clinical responses at 2 years in SpA patients starting therapy with SEC versus TNFis in routine clinical care.
Methods: This was an analysis from UCRCR Registry, which is a prospective single center study. For the present study we analyzed all consecutive patients with axial (AxSpA) or peripheral SpA (pSpA), including psoriatic arthritis (PsA) starting or switching bDMARD therapy, either a TNFi or SEC from 1/2016 till 12/2022. We excluded patients with IBD-related SpA, patients starting ustekinumab (or apremilast) and patients switching from a bio-originator to a biosimilar TNFi or vice versa. We compared disease activity scores improvement at 6 months using linear regression analysis and treatment retention using Kaplan-Meier survival curves with log-rank test and Cox regression, adjusting for diagnosis (AxSpA vs pSpA,), presence of psoriasis, age, sex, disease duration, treatment line, co-administered csDMARDs (yes/no), smoking status (ever/never) and rheumatic disease comorbidity index (RDCI).
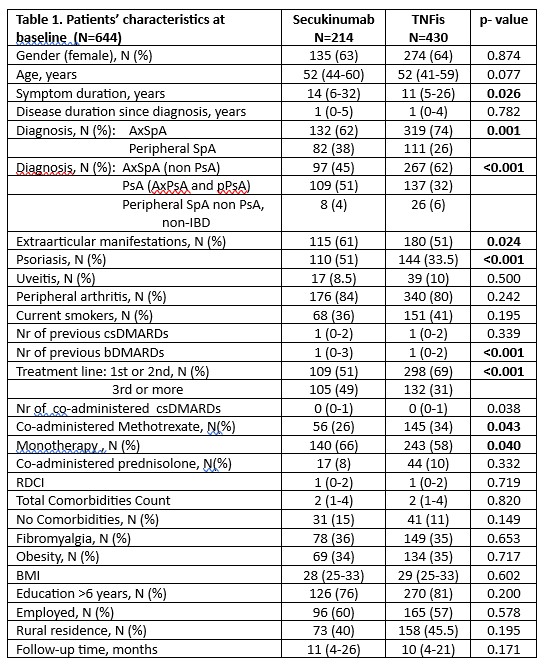
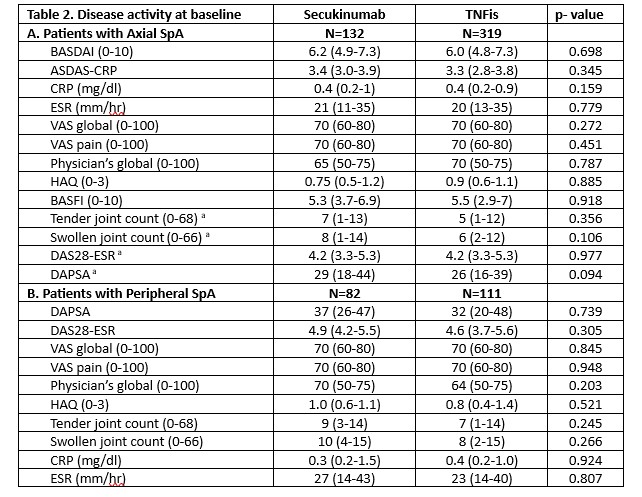
Results: A total of 644 patients with SpA started/switched bDMARD (SEC:214, TNFis:430). SEC was the ≥3rd bDMARD in 49% of patients compared to 31% of TNFis (p< 0.001). AxSpA was the diagnosis in 62% patients starting SEC and 74% starting TNFis while psoriasis was more common with SEC (51% vs 33.5% in TNFis). Monotherapy was more common with SEC compared to TNFis (66% vs 58%, p=0.04). All other patients’ characteristics at baseline, including extraarticular manifestations (except psoriasis), comorbidities’ indices and sociodemographics were similar in the two groups. Baseline disease activity in both AxSpA and peripheral SpA was also comparable in patients starting SEC and TNFi.
Unadjusted 2-year treatment retention was higher in patients receiving SEC compared to TNFi both overall (SEC: 60%, TNFi: 45%, p=0.004) and separately in AxSpA (SEC: 59.5%, TNFi: 48%, p=0.039) and pSpA patients (SEC: 61%, TNFi: 36.5%, p=0.020).
In adjusted analysis, SEC administration was an independent predictor for higher bDMARD retention overall [HR (95%CI)=0.61 (0.45-0.84), p=0.002] and separately in AxSpA [HR=0.65 (0.45-0.95), p=0.025] and in pSpA [HR=0.54 (0.31-0.93), p=0.028].
Mean BASDAI and ASDAS improvements at 6, 12, 18 and 24 months of treatment were similar in patients with AxSpA receiving SEC or TNFis [at 6 months, mean(SD) δBASDAI:0.9 (2.2) vs 1.3 (2.2) respectively (p=0.49) and δASDAS: 0.5 (0.9) vs 0.6 (1.0), p=0.75]. Similarly, in patients with pSpA, δDAPSA was comparable in the two groups at all timepoints [at 6 months, mean (SD)δDAPSA: 12.3 (21) vs 8.1 (11), p=0.294]. Adjusted analyses provided similar results to the unadjusted analyses in both axial and peripheral SpA.
Conclusion: In real-world SpA patients, administration of Secukinumab results in similar clinical responses but higher treatment retention compared to TNFis.
To cite this abstract in AMA style:
Flouri I, Repa A, Avgoustidis N, Pitsigavdaki S, Pateromichelaki K, Terizaki M, Bertsias G, Sidiropoulos P. Two-Year Treatment Adherence and Clinical Response to Secukinumab Compared to TNF Inhibitors in Axial and Peripheral Spondyloarthritis Patients: A Single-Center Prospective Observational Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/two-year-treatment-adherence-and-clinical-response-to-secukinumab-compared-to-tnf-inhibitors-in-axial-and-peripheral-spondyloarthritis-patients-a-single-center-prospective-observational-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/two-year-treatment-adherence-and-clinical-response-to-secukinumab-compared-to-tnf-inhibitors-in-axial-and-peripheral-spondyloarthritis-patients-a-single-center-prospective-observational-study/