Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: In Chile, patients with rheumatoid arthritis (RA) can apply to receive state-funded biological or JAK inhibitor treatment if they have failed (defined as a DAS28-ESR persistently greater than 5.1) to the combination of three synthetic DMARDs (including methotrexate, leflunomide, sulfasalazine, and/or hydroxychloroquine) used for at least 6 months. Our objective was to evaluate the two-year persistence of the first biologic or JAK inhibitor used in this group of patients.
Methods: We conducted a retrospective evaluation of patients benefiting from state-funded biological or JAK inhibitor treatments at two healthcare centers. Demographic variables were recorded, including gender, age, comorbidities (Charlson Comorbidity Index), years since RA diagnosis, synthetic DMARDs used at the time of starting biological or JAK inhibitor treatment, use of corticosteroids and NSAIDs, the specific biological or JAK inhibitor used, persistence with the medication after two years, and, for those who discontinued treatment, the cause of discontinuation and duration of persistence. The relationship between persistence and different demographic and baseline variables was evaluated using logistic binary regression.
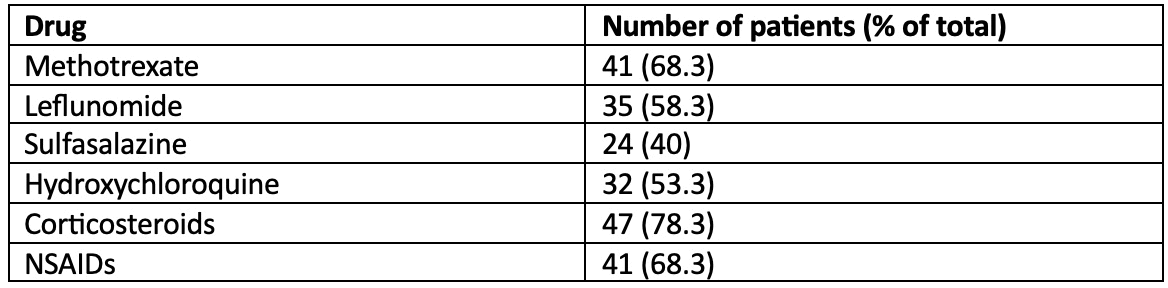
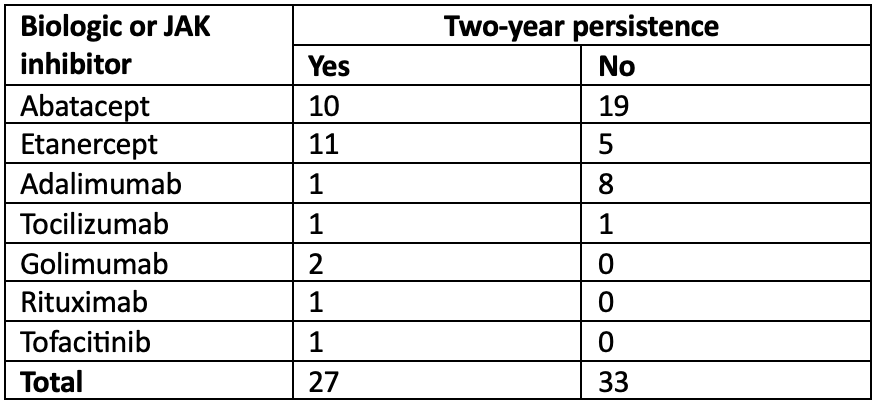
Results: We included 60 patients, of whom 52 were women (86.7%). The mean age was 58 years (standard deviation 12.5), and the Charlson Comorbidity Index was 3 or higher in 40% of patients. The median baseline DAS28-ESR was 5.8 (interquartile range 5.4-6.7), and the median years since diagnosis was 10.5 (IQR 5.7-16.5). Table 1 provides the distribution of synthetic DMARDs, corticosteroids, and NSAIDs used at the start of biologic or JAK inhibitor treatment. Table 2 presents the first-line biologic or JAK inhibitor used and its persistence. At the two-year mark, 27 patients (45%) persisted with the first-line biologic or JAK inhibitor. Among the 33 patients who discontinued treatment, the mean duration of persistence was 323 days (SD 176). The univariate analysis revealed significant associations between persistence and the use of baseline sulfasalazine (62.5% of users persisted at 2 years, compared to 33.3% of non-users), as well as with the specific first biologic or JAK inhibitor used (see Table 2). The primary cause of treatment discontinuation was loss of efficacy (60.6%), followed by adverse effects (24.2%).
Conclusion: The two-year persistence rate of the first biologic or JAK inhibitor in this group of patients with long-standing rheumatoid arthritis, high comorbidity index, and failure of synthetic DMARDs triple therapy was low, at only 45%. Loss of efficacy was the main reason for treatment discontinuation. A trend towards lower persistence with the use of adalimumab was observed, but this finding should be interpreted with caution as adalimumab was the only biologic that underwent exchanges between biosimilars and the original molecule. The association between greater persistence and the initial use of sulfasalazine should be explored in future studies. Notably, the utilization of JAK inhibitors as first-line advanced therapy was very low.
To cite this abstract in AMA style:
Ibanez Vodnizza S, Poblete M, Valenzuela M, Canals M, García D, Jaque C. Two-Year Persistence of First-Line Biological or JAK Inhibitor Treatment in Patients with Long-Standing Rheumatoid Arthritis and Failure of Triple Therapy with Synthetic DMARDs [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/two-year-persistence-of-first-line-biological-or-jak-inhibitor-treatment-in-patients-with-long-standing-rheumatoid-arthritis-and-failure-of-triple-therapy-with-synthetic-dmards/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/two-year-persistence-of-first-line-biological-or-jak-inhibitor-treatment-in-patients-with-long-standing-rheumatoid-arthritis-and-failure-of-triple-therapy-with-synthetic-dmards/