Session Information
Date: Sunday, November 8, 2020
Title: Pediatric Rheumatology – Clinical Poster II: Systemic JIA, Autoinflammatory, & Scleroderma
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: The treatment of systemic juvenile idiopathic arthritis (SJIA) has changed dramatically over the past decade, associated with overall improvement in functional outcomes. There may be an early “window of opportunity” where biologic therapies provide maximal benefit while decreasing morbidity associated with glucocorticoids (GC). The Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry has enrolled more than 10,000 patients with pediatric rheumatic diseases. Our aim was to (1) assess temporal trends in the timing of non-biologic and biologic medication use in the first year after diagnosis of SJIA with an emphasis on early initiation of biologics, and (2) determine if demographic factors impact timing of biologic introduction.
Methods: Patients with SJIA enrolled in the CARRA registry between 2015 and 2018 were included. Medication information was collected retrospectively for all patients at enrollment in the Registry and prospectively after enrollment. Patients with missing month of diagnosis or medication start date were excluded. Medications were grouped by mechanism of action, and temporal trends in medication class usage were assessed using frequencies. Timing of initiation of IL-1 inhibition (IL-1i) and IL-6 inhibition (IL-6i) were assessed by year of diagnosis. Patients were grouped into one of three categories: (1) initiated IL-1i or IL-6i in first 91 days, (2) initiated on day 92-364, and (3) no use in first year. Sub-analysis was performed looking at the timing of IL-1/6i initiation (0-3, 3-6, 6-12 months after diagnosis) was analyzed to determine if demographic factors contribute to timing of medication start.
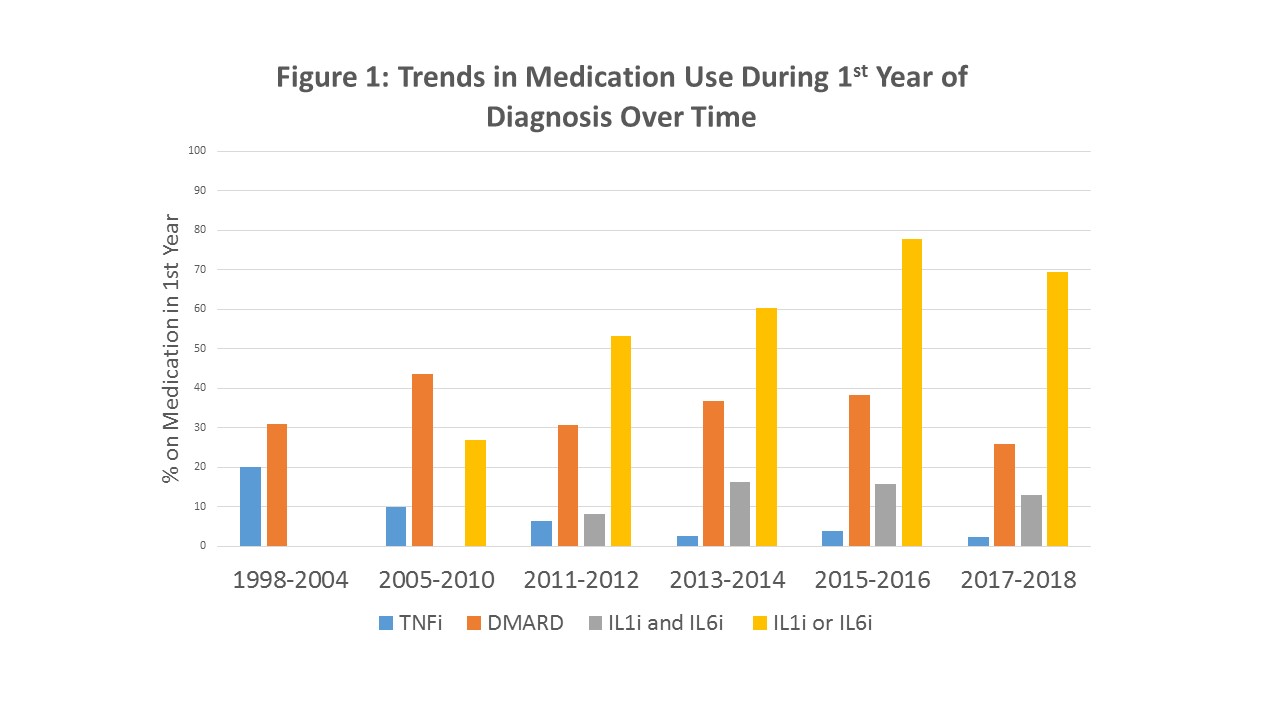
Results: 561 patients were included in the analysis. There was an upward trend in IL-1/6i usage during the first year of treatment over time (Figure 1), with a concurrent decline in tumor necrosis factor inhibitor (TNFi) use. Nearly half of patients (46%) were treated with IL1/6i in the first 3 months and 59.4% were treated with IL-1/6i in the first year. Initiation of IL-1i and IL-6i in the first 91 days following diagnosis appears to have increased over time (Figure 2). No significant differences were found in timing of introduction of IL-1/6i based on age, sex, race, or median time from symptom onset to diagnosis. Timing of IL-1/6i introduction did not affect frequency of DMARD use in the first 12 months (Table 1).
Conclusion: In SJIA patients in the CARRA Registry enrolled over a 3-year period, there has been an increase in IL-1/6i usage. Of those prescribed IL-1/6i, the majority began treatment in the first three months of disease. This does not appear to vary by age of diagnosis, time from symptom onset to diagnosis, race/ethnicity, or methotrexate use. Our data suggests that there may be an increase in “early” use of IL-1i and IL-6i, possibly due to increasing provider comfort, experience, and availability, as well as the perceived potential for a “window of opportunity” in treatment and desire to decrease GC use in these patients. Whether these changes result in improved disease outcomes requires further study.
To cite this abstract in AMA style:
Janow G, Beukelman T, Kimura Y, Schneider R, Mohan S, Rodich G, Son M. Trends in Timing of Biologic Use for Treatment of Systemic Juvenile Idiopathic Arthritis in the CARRA Registry [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/trends-in-timing-of-biologic-use-for-treatment-of-systemic-juvenile-idiopathic-arthritis-in-the-carra-registry/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/trends-in-timing-of-biologic-use-for-treatment-of-systemic-juvenile-idiopathic-arthritis-in-the-carra-registry/