Session Information
Date: Sunday, November 13, 2022
Title: RA – Treatment Poster III
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: ABP 501 is the first adalimumab (ADA) biosimilar approved by the European Medical Association and the Food and Drug Administration for the treatment of immune-mediated inflammatory diseases including RA, PsA, and AS. Before ABP 501 becomes available in the US, real-world evidence from European countries on utilization patterns of ABP 501 can provide useful information, especially regarding indications such as PsA and AS which were approved on the basis of extrapolation.
Methods: This retrospective cohort analysis using the IQVIA German pharmacy claims database included patients (≥ 18 years) who initiated ABP 501 between October 2018 and March 2020 and had ≥365 days of continuous observation both before and after initiation of ABP 501. Patient characteristics and medication use at baseline (within 12 months before the initiation of ABP 501) were reported for each indication and stratified by prior use of ADA products. Persistence was evaluated using Kaplan-Meier analysis, with a permissible treatment gap of up to 120 days. Initial switching patterns after discontinuation of ABP 501 were also assessed. This study was a descriptive analysis, and no statistical comparisons were conducted.
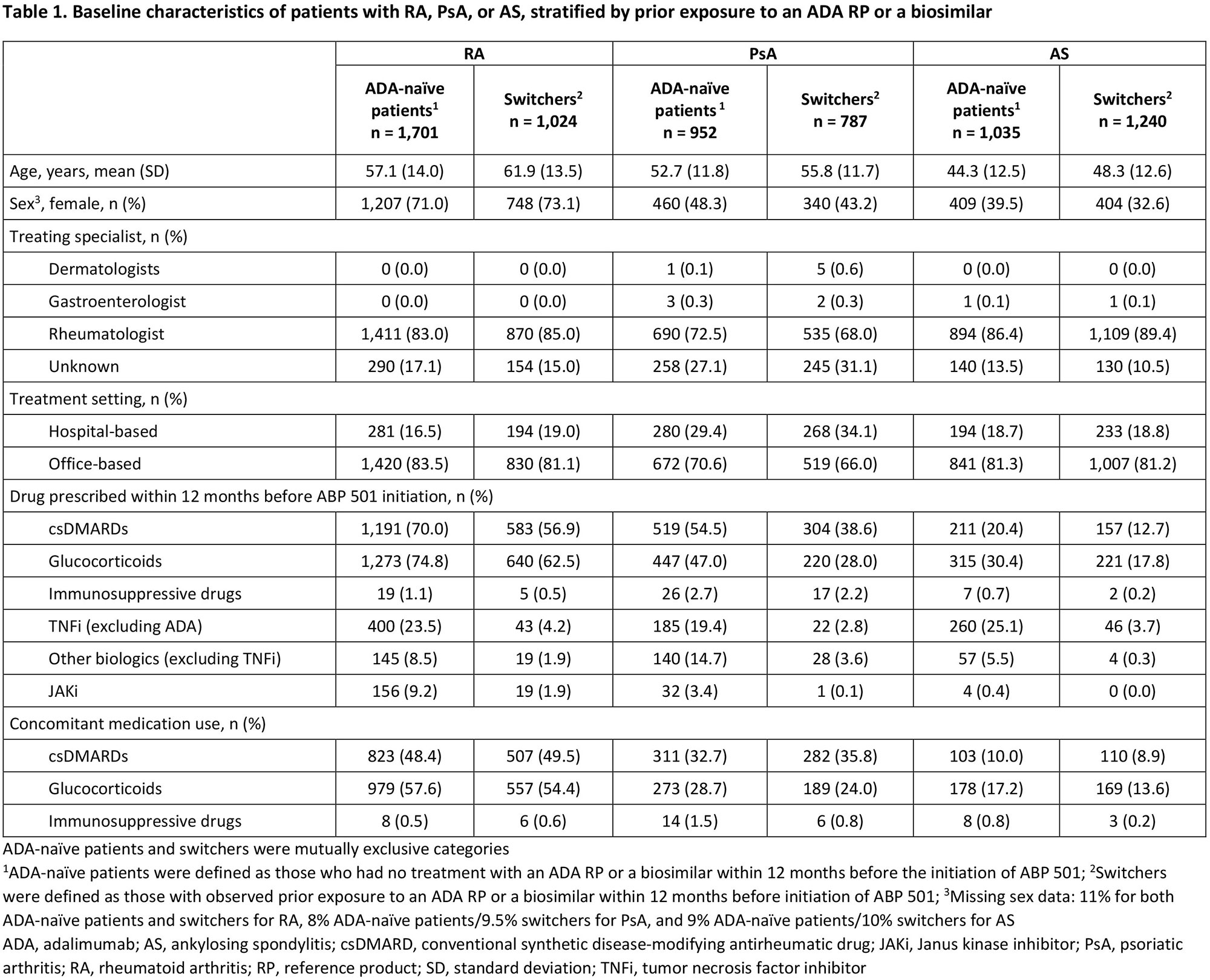
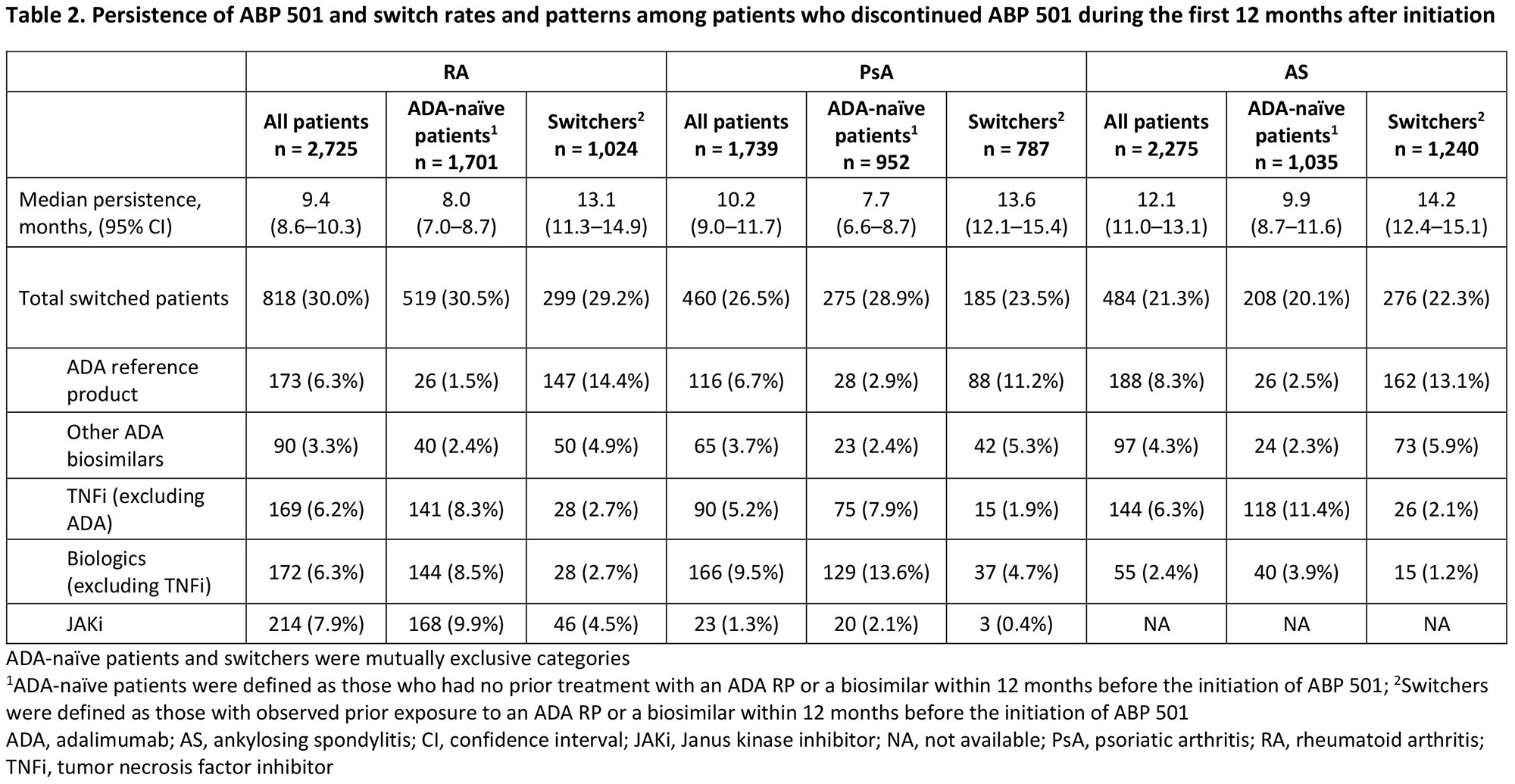
Results: The analysis included 6,739 patients (RA, n=2,725; PsA, n=1,739; AS, n=2,275) comprising patients with (switchers) or without (ADA-naïve patients) prior exposure to an ADA reference product (RP) or an ADA biosimilar. Patient demographics and treating specialists/settings are presented in Table 1. Prior use of conventional synthetic DMARDs, glucocorticoids, other biologics (including non-ADA TNF inhibitors [TNFi]), and Janus kinase inhibitors (JAKi) during the baseline period was observed in all patients and was numerically higher in ADA-naïve patients than in switchers across indications (Table 1). Median (95% CI) persistence on ABP 501 was 9.4 (8.6–10.3), 10.2 (9.0–11.7), and 12.1 (11.0–13.1) months for patients with RA, PsA, and AS, respectively, and was consistently longer for switchers than for ADA-naïve patients across indications (Table 2). For each indication, the overall switching rate was similar between Switchers and ADA-naïve patients in the first 12 months after initiating ABP 501, with the highest rate observed among patients with RA (30.0%) and the lowest rate among those with AS (21.3%). Switching patterns varied by indication, with the most common medication switched to being JAKi for RA (7.9%), other non-TNFi biologics for PsA (9.5%), and ADA RP for AS (8.3%) (Table 2). Switching to ADA RP was observed more frequently among switchers (RA: 14.4%, PsA: 11.2%, AS: 13.1%) than among ADA-naïve patients (RA: 1.5%, PsA: 2.9%, AS: 2.5%) (Table 2).
Conclusion: Numerical differences in persistence and switching patterns were observed between ADA-naïve patients and switchers. Thus, differences in baseline medication use and available treatment options for each indication should be considered when interpreting persistence and switching pattern data. Further studies are necessary to better understand the reasons for these differences in switching patterns.
To cite this abstract in AMA style:
Jin R, Kruppert S, Scholz F, Hammer M, Kricorian G, Collier D, Kay J. Treatment Persistence and Switching Patterns of ABP 501 (AMGEVITA®) in German Patients with Rheumatic Diseases [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/treatment-persistence-and-switching-patterns-of-abp-501-amgevita-in-german-patients-with-rheumatic-diseases/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-persistence-and-switching-patterns-of-abp-501-amgevita-in-german-patients-with-rheumatic-diseases/