Session Information
Date: Tuesday, November 9, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster III: Psoriatic Arthritis II (1801–1835)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Treatment of non-biologic-DMARD-IR (DMARD-IR) PsA patients with upadacitinib (UPA) at 15 mg QD, an oral JAK1 selective inhibitor, resulted in improvement in musculoskeletal symptoms, psoriasis, physical function, fatigue, quality of life, and inhibited radiographic progression; improvements were observed as early as Week 2 (ACR20 and ACR50). UPA 15 mg QD was non-inferior to ADA 40 mg EOW for ACR20.1 The objective of this analysis was to determine the relative biological pathway modulation of UPA compared with ADA in patients with PsA via the evaluation of a pre-defined set of plasma proteins associated with inflammation.
Methods: Patients from the SELECT-PsA 1 study (DMARD-IR PsA patients) were randomly selected (PBO, n=100; UPA 15 mg QD, n=100; ADA 40 mg EOW, N = 100). The levels of 92 inflammation related protein biomarkers (pBM) were analyzed using the Olink® platform in plasma samples collected at baseline, week 2, and 12; change from baseline in protein levels were expressed as Log2 Fold Change; a Repeated Measure Mixed Linear Model identified proteins modulated by UPA and ADA compared to Baseline. Functional pathway prediction was performed with Ingenuity® Pathway Analysis (Qiagen Inc.) where 52 significantly modulated pBM (mean |Log2 FC| ≥ 0.1 AND FDR ≤ 0.05) were selected; results were summarized based on 3 core biological groups: 1) adaptive immune system, 2) innate immune system, and 3) non-immune connective and vascular systems.
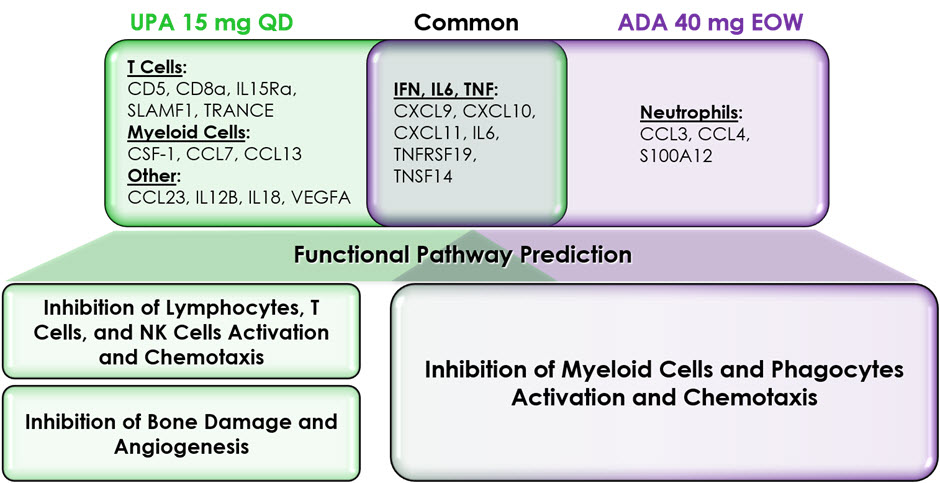
Results: At the single pBM-level, at the week 2 and 12 time points, treatment with UPA 15 mg QD resulted in distinct down modulation of T cell-associated (CD5, CD8a, IL15Ra, SLAMF1, TRANCE) and myeloid cell-associated pBM (CSF-1, CCL7, CCL13) that was not observed in the ADA treated group. Reciprocally, treatment with ADA 40 mg EOW resulted in a specific down modulation of a subset of neutrophil associated pBM (CCL3, CCL4, and S100A12). Both treatments resulted in the down modulation of IFN-, IL6-, and TNF-related pBM (CXCL9, CXCL10, CXCL11, IL6, TNFRSF19, and TNSF14 ) suggesting a common node of activity related to these pivotal cytokine-signaling pathways.
Functional pathway prediction based on the pBM data revealed that treatment with UPA is preferentially associated with the inhibition of T cells, but also NK cells and lymphocytes, compared to the predicted effects of treatment with ADA. Treatment with UPA also preferentially inhibited pathways related to bone damage and angiogenesis, as compared to the predicted effect of treatment with ADA. Finally, both treatments were predicted to inhibit multiple pathways associated with the activity of myeloid cells and phagocytes.
Conclusion: Consistent with previous observations in RA2, UPA is predicted to inhibit multiple functional pathways associated the pathobiology of PsA belonging to the general categories of adaptive and innate immunity but also non-immune vascular and connective tissue biology. In contrast, treatment with ADA appears to affect more specifically functional pathways associated with the innate immune system.
References:
1. McInnes, I. et al. Annals of the Rheumatic Diseases 79, 16-17 (2020).
2. Sornasse, T., Song, I.H., Radstake, T. & McInnes, I. Annals of the Rheumatic Diseases 79, 581-582 (2020).
To cite this abstract in AMA style:
Sornasse T, Anderson J, Kato K, Lertratanakul A, Ritchlin C, McInnes I. Treatment of Non-biologic-DMARD-IR PsA Patients with Upadacitinib or Adalimumab Results in the Modulation of Distinct Functional Pathways: Proteomics Analysis of a Phase 3 Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/treatment-of-non-biologic-dmard-ir-psa-patients-with-upadacitinib-or-adalimumab-results-in-the-modulation-of-distinct-functional-pathways-proteomics-analysis-of-a-phase-3-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-of-non-biologic-dmard-ir-psa-patients-with-upadacitinib-or-adalimumab-results-in-the-modulation-of-distinct-functional-pathways-proteomics-analysis-of-a-phase-3-study/