Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Lorecivivint (LOR), an intra-articular CLK/DYRK inhibitor thought to modulate inflammatory and Wnt pathways, demonstrated beneficial effects on clinical and radiographic outcomes in previous knee OA clinical trials. While clinical outcomes focus on differences between treatment groups, an individual’s “acceptable symptom state” may provide a meaningful and clinically relevant assessment. Year 1 results from a 2-year Phase 3 extension trial, OA-07 (NCT04520607), evaluating LOR 0.07 mg safety and efficacy with WOMAC Pain [0-100] and Function [0-100] subscales, are reported here within context of the Patient Acceptable Symptom State (PASS). The objective of this analysis was to evaluate long-term, repeated LOR treatment of severe knee OA patients as assessed by PASS.
Methods: Patients with structurally advanced knee OA (medial joint space width [JSW] 1.5-4.0 mm) who completed the parent Phase 3 trial (OA-11, NCT 03928184) were eligible for enrollment into OA-07. A repeat injection according to the original OA-11 trial randomization (LOR / placebo (PBO)) was given at the beginning of the OA-07 trial’s single-blind (for patients and investigators) phase. PASS was assessed annually at the start and the end of OA-07 Year 1, irrespective of symptom state, using a ‘yes/no’ response to the question “Taking into account all the activities you have during your daily life, your level of pain, and also your functional impairment, do you consider that your current state is satisfactory?” PASS response and odds ratios (OR; 95% CI) between treatment groups were calculated using baseline-adjusted logistic regression. Differences between treatment groups were explored for those patients with a positive PASS response.
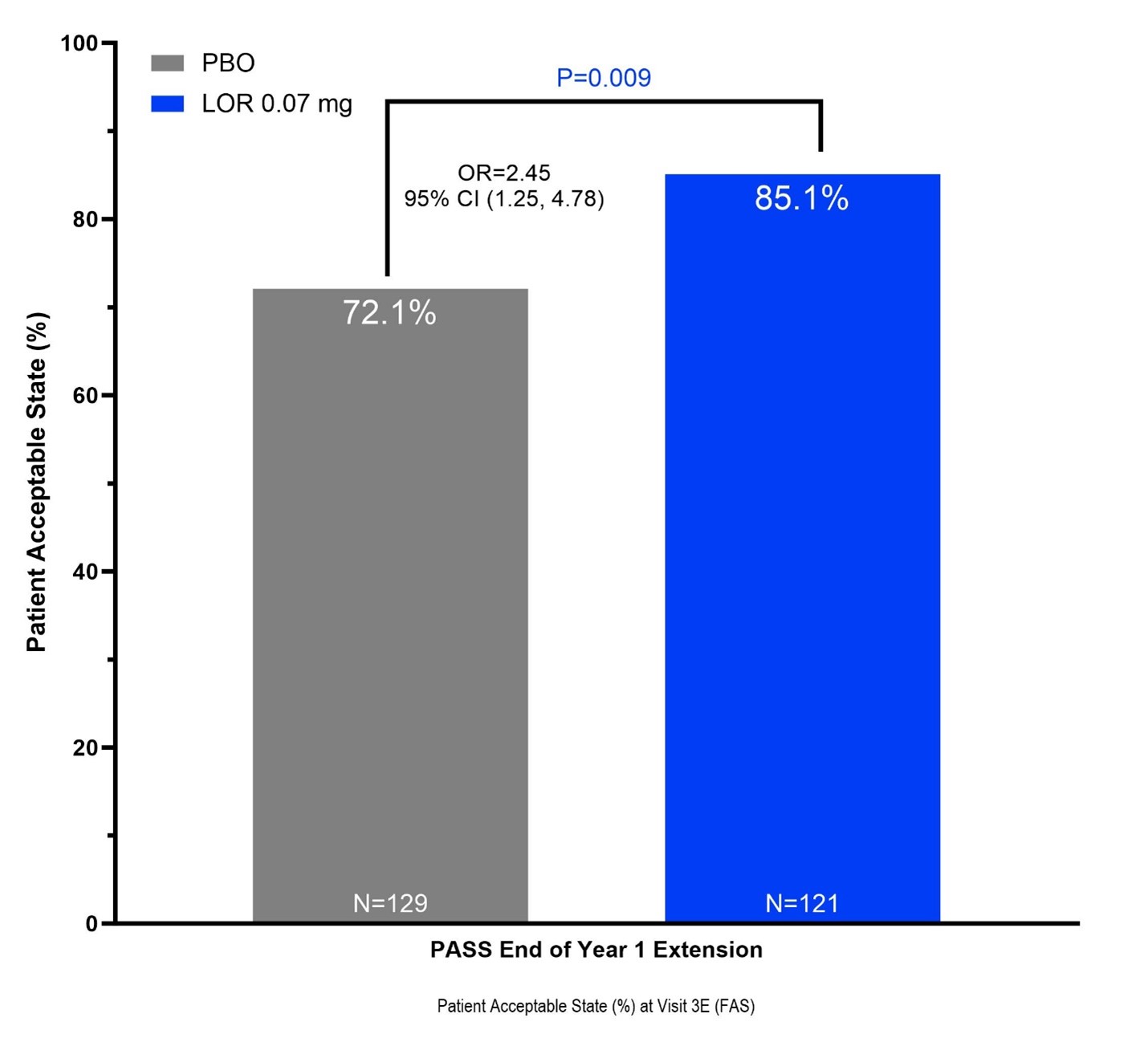
Results: 276 patients (mean age 61.0 ± 8.2 years, BMI 31.8 ± 4.9 kg/m2, female 62.7%, KL3 45.3%, medial JSW 2.63 ± 0.69 mm, 67.4% bilaterally symptomatic, 68.5% medial JSW < 3 mm) were enrolled. LOR appeared safe and well-tolerated with no major adverse event rate differences compared to PBO during OA-07 Year 1. Treatment with LOR significantly increased the odds of reporting a ‘yes’ PASS response at the end of Year 1 (LOR 103 of 121 (85.1%) vs PBO 93 of 129 (71.1%), OR 2.45 [1.25, 4.78], P=0.009). Patients with a positive PASS response also reported greater improvement in WOMAC Pain (LOR -6.8 (± 2.0) vs PBO -1.0 (± 2.1), t-test P=0.049) and WOMAC Function (LOR -7.0 (± 1.9) vs PBO -2.4 (± 1.9), t-test P=0.090) at the end of Year 1 following the repeat injection at the start of the OA-07 extension study.
Conclusion: In this analysis of a phase 3 extension study of LOR compared to PBO, LOR-treated patients were significantly more likely to achieve a positive patient acceptable symptom state 12 months after a second injection. Pain and function outcomes showed greater improvement for those subjects reporting an acceptable symptom state. These data suggest that multiple once-yearly doses of LOR may provide long-term benefits for knee OA symptoms.
To cite this abstract in AMA style:
Swearingen C, Yazici Y, Tambiah J, Conaghan P. Treatment of Advanced Knee OA with Lorecivivint Leads to Improved Long-Term Patient Acceptable Symptom State (PASS) Compared to Placebo: Data from Phase 3 Extension Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/treatment-of-advanced-knee-oa-with-lorecivivint-leads-to-improved-long-term-patient-acceptable-symptom-state-pass-compared-to-placebo-data-from-phase-3-extension-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-of-advanced-knee-oa-with-lorecivivint-leads-to-improved-long-term-patient-acceptable-symptom-state-pass-compared-to-placebo-data-from-phase-3-extension-trial/