Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Although the increasing availability of biologic therapies has significantly improved outcomes for patients with JIA, a substantial proportion of patients require switching from the initial biologic (b)DMARD to a different bDMARD or a targeted synthetic (ts)DMARD. Limited data are available to inform choice of switching to a second TNFi versus bDMARD with a different mechanism. We compared the effectiveness of switching to a second TNF inhibitor (TNFi) versus a bDMARD with a different mechanism of action or tsDMARD in children with insufficient response to initial TNFi.
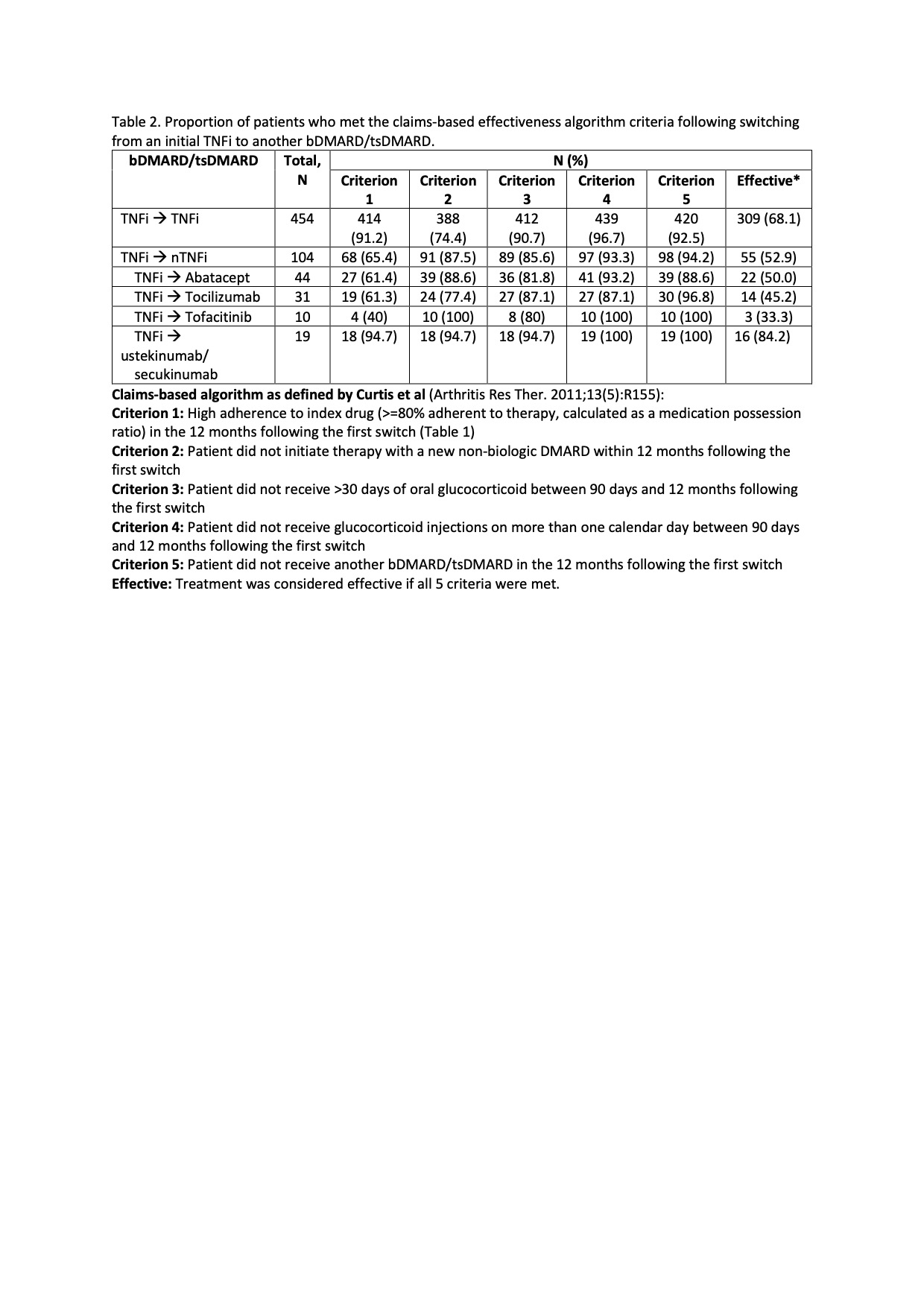
Methods: We conducted a retrospective study of a national insurance database in the US (years 2008 to 2020). Study subjects included children and adolescents (aged < 19 years) who initiated a TNFi and then subsequently switched to another bDMARD/tsDMARD therapy, had ≥2 medical claims with a JIA diagnostic code, had been enrolled in the health plan for ≥6 months prior to starting initial TNFi, continued enrollment ≥365 days following switching to a subsequent bDMARD/tsDMARD therapy, and did not have claims associated with systemic JIA. Effectiveness of the second therapy was estimated using a previously validated claims-based algorithm (Curtis) (Table 2). Inverse probability weighting was applied to estimate treatment effectiveness.
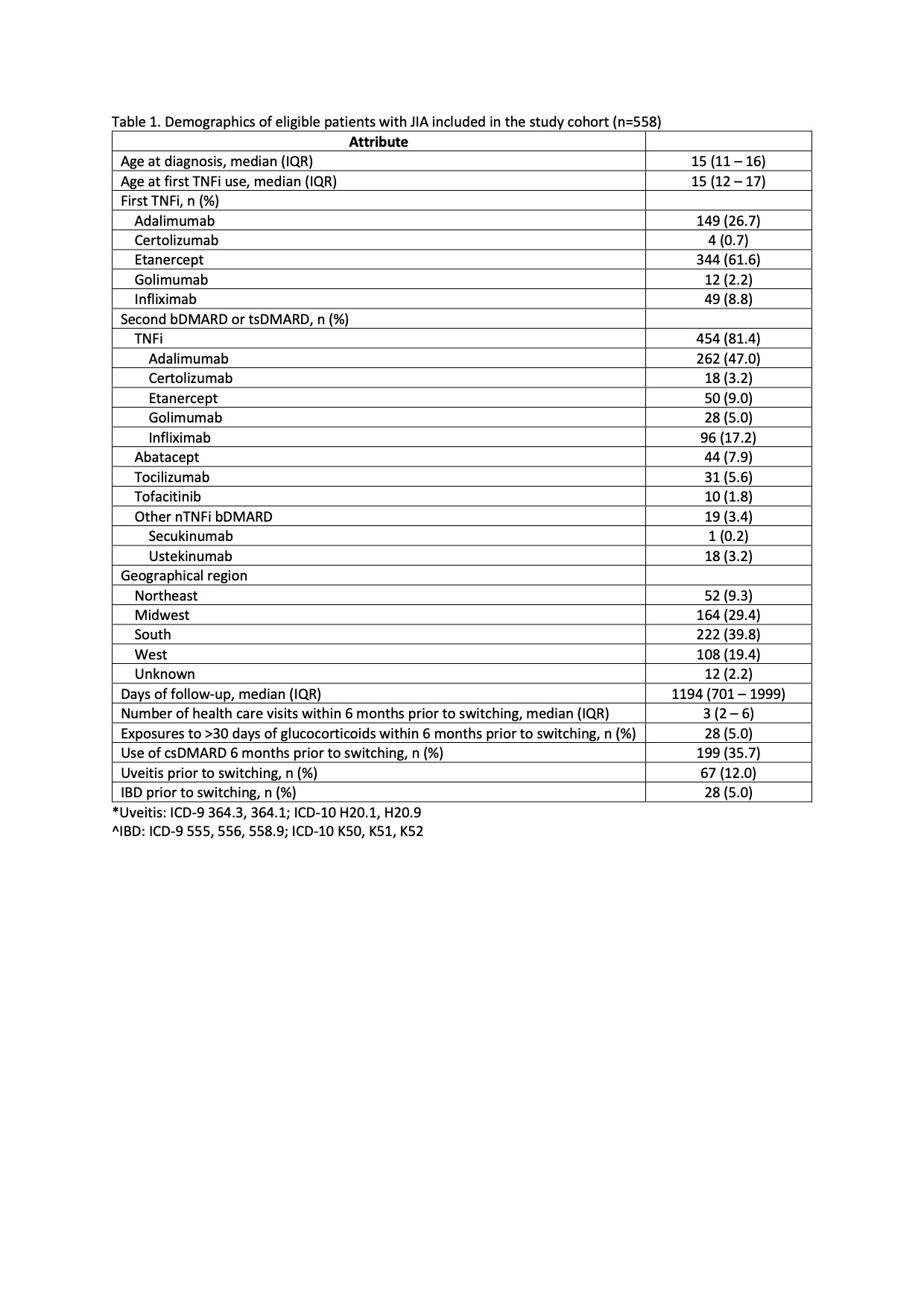
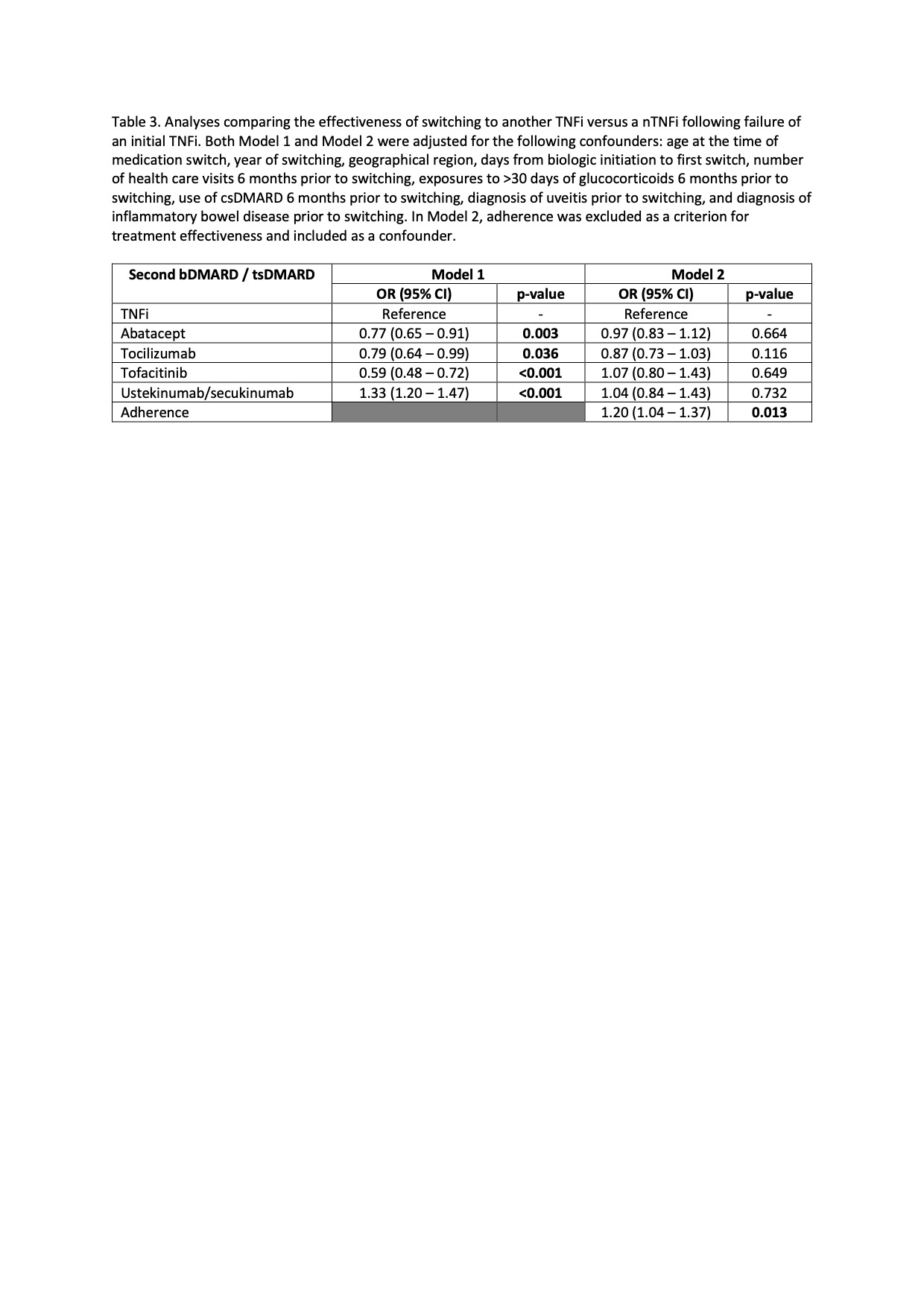
Results: 558 eligible children were included in the study (Table 1). The median follow-up was 1194 days (IQR 701-1999). During this period, 81% (n=454) of patients switched from an initial TNFi therapy to another TNFi, while the remaining switched to a different bDMARD/tsDMARD, most commonly abatacept (n=44) or tocilizumab (n=31)(Table 2). Ustekinumab/secukinumab were the most effective second therapy, followed by a second TNFi, with 84% and 68% of patients responding to the therapy, respectively. Compared to switching to another TNFi, switching to ustekinumab/secukinumab was significantly more effective (OR 1.33; 95% CI 1.20-1.47). However, abatacept (OR 0.77; 95% 0.65-0.91), tocilizumab (OR 0.79; 95% CI 0.64-0.99), and tofacitinib (OR 0.59; 95% CI 0.48-0.72) were less effective as a second therapy compared with a second TNFi (Table 3, Model 1). Further analysis revealed that adherence to the second therapy (criterion 1 of the treatment effectiveness algorithm) differed significantly between patients who switched to non-TNFi (nTNFi) vs a second TNFi (65% vs 91% respectively, Table 2), with the exception of ustekinumab/secukinumab as nTNFi. Omitting adherence as a criterion for defining treatment effectiveness and instead including it as a covariate showed that treatment effectiveness did not differ by medication classes. Adherence was significantly associated with treatment effectiveness (OR 1.20; 95% CI 1.04 – 1.37).
Conclusion: Our study showed no differences in treatment effectiveness of a second TNFi compared with another b/ts DMARD following initial TNFi therapy. Notably, medication adherence was the only significant factor affecting treatment effectiveness. Further studies are needed to understand the variation in adherence rate for patients with JIA switching to a second b/ts DMARD.
To cite this abstract in AMA style:
Ong M, Ringold S, Mannion M, Natter M, Kimura Y. Treatment Effectiveness Following Switching from Initial TNF Inhibitor in Juvenile Idiopathic Arthritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/treatment-effectiveness-following-switching-from-initial-tnf-inhibitor-in-juvenile-idiopathic-arthritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-effectiveness-following-switching-from-initial-tnf-inhibitor-in-juvenile-idiopathic-arthritis/