Session Information
Date: Friday, November 6, 2020
Title: Patient Outcomes, Preferences, & Attitudes Poster I: RA, Spondyloarthritis, & OA
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Biologic disease-modifying antirheumatic drug (bDMARD) therapy has been shown to be effective in the treatment of axial spondyloarthritis (axSpA).1,2 Little is understood about axSpA patients’ treatment journey from their own perspective. The purpose of this study was to explore treatment decision-making of axSpA patients and to identify barriers and facilitators to treatment optimization.
Methods: This was an observational, cross-sectional study of participants (pts) in the ArthritisPower registry using a survey developed in partnership with patients and clinicians. Pts aged ≥ 19 years with physician-diagnosed axSpA and access to a computer/smartphone were invited to complete electronic patient-reported outcome measures and an online survey on treatment (tx) decision making. Pts’ responses were classified into 2 groups based on whether or not currently on a bDMARD.
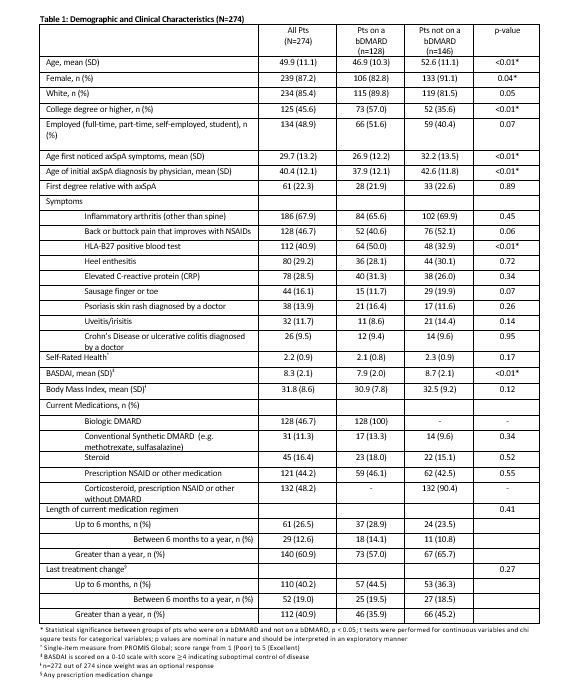
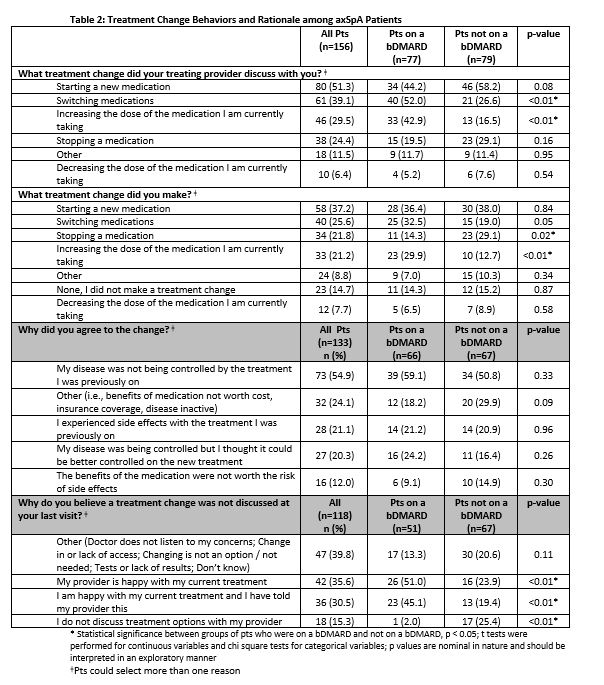
Results: 274 pts met inclusion criteria of whom 87.2% were female, 85.4% White, with mean age of 50 years (Table 1). The majority of pts (82.1%) had been diagnosed with axSpA by a rheumatologist. Of all pts, 56.9% (n=156) discussed a tx change at their most recent physician visit, 79.5% of whom researched the treatment change on their own, and 46.2% (n=72) of these pts reported having raised the issue with their clinician themselves. Half (51.3%) of tx changes discussed were related to initiation of a new medication, compared with stopping a medication (24.4%) and/or changing its dose (35.9%). The majority of pts (85.3%; n=133) who discussed a change agreed to the tx change and most pts agreeing to a change did so because they felt their disease was not being controlled (54.9%; n=73) or because they thought control could be better on new tx (n=27; 20.3%). Most pts (62.8%) reported agreeing to the suggested tx change by end of clinic visit, while 15.4% needed ≤ 1 week to decide. Pts on a bDMARD were more likely to discuss switching medications (52.0%) or increasing dose (42.9%) vs. non-bDMARD pts (26.6%, p=0.0012; 16.5%, p=0.0003) (Table 2). Among the pts (n=23) who declined a tx change, top reasons were pts not believing there were more efficacious options than their current tx (60.9%) and worries about potential side effects of a new tx (56.5%). For bDMARD pts whose tx change was not discussed at the last visit, pts felt it was because either they or their provider were happy with the current tx (Table 2); bDMARD-treated pts and their clinicians were more likely to report satisfaction with current tx as the reason a tx change was not discussed compared to non-bDMARD pts. Overall, factors that pts on bDMARDs (n=128) considered most important when making tx decisions were preventing other long-term consequences of untreated axSpA (92.2%), preventing damage from axSpA (89.1%), and advice from their doctor (87.5%) (Table 3).
Conclusion: Most axSpA patients reported discussing tx changes with their provider at their most recent visit and had done their own research about it beforehand. Prevention of long-term damage and doctor’s advice are major factors influencing patient decisions about tx.
References:
[1] Dubash S, et al. Ther Adv Chronic Dis. 2018;9(3):77–87.
[2] Van Der Heijde D, et al. Ann Rheum Dis. 2017;76(6):978–91.
To cite this abstract in AMA style:
Nowell W, Hunter T, Gavigan K, Curtis J, Malatestinic W, Bolce R, Lisse J, Kronbergs A, Himelein C, Walker J, Walsh J. Treatment Decision Making Among Axial Spondyloarthritis Patients: Real-World Data from the ArthritisPower Registry [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/treatment-decision-making-among-axial-spondyloarthritis-patients-real-world-data-from-the-arthritispower-registry/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-decision-making-among-axial-spondyloarthritis-patients-real-world-data-from-the-arthritispower-registry/