Session Information
Date: Tuesday, October 23, 2018
Title: Rheumatoid Arthritis – Treatments Poster III: Biosimilars and New Compounds
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: The number of biosimilars approved for the treatment of rheumatoid arthritis (RA) is constantly increasing. Until now, there are just a few analyses investigating retention rates of biosimilars and the respective originators. We compared treatment survival on SB4 to the originator etanercept (oETN) using real-world data.
Methods: We used data gathered until April 2018 from the prospective, longitudinal RABBIT (Rheumatoid Arthritis: Observation of biologic therapy) cohort. RA patients are enrolled in RABBIT when they start a biologic, biosimilar or new csDMARD treatment. Bionaive patients enrolled since 2015 starting a treatment with either SB4 or oETN who had at least one follow-up visit were compared. The drug survival rates during the first six months were analyzed using Kaplan-Meier curves.
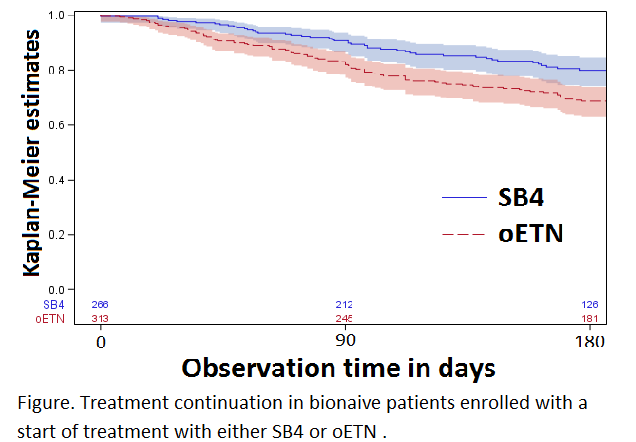
Results: Overall, 266 patients treated with SB4 and 313 with oETN fulfilled the inclusion criteria. Compared to oETN patients, those enrolled with SB4 had lower disease duration (7.5 vs. 8.7 years), were younger (58 vs. 59 years) and significantly fewer patients had three or more comorbidities (39% vs. 47%). Kaplan-Meier curves show higher retention rates over 6 months for SB4 than oETN (figure). Patients switching from oETN to SB4 or vice versa were censored and not counted as discontinuations (oETN to SB4: n=7, SB4 to oETN: n=1). Adjusting for disease duration and comorbidities had no significant influence on the results. 9% (n=25) of SB4 patients and 18% (n=55) of oETN patients stopped treatment during the first 90 days. Additional 8% (n=21, SB4) / 12% (n=37, oETN) stopped the treatment within 180 days after enrolment. The most frequent reasons for discontinuation of both treatments were adverse events (AE) in 48% (21 of 44, SB4) / 54% (49 of 91, oETN) and ineffectiveness in 36% (16 of 44, SB4) / 36% (33 of 91, oETN) of patients. The most frequent AE leading to discontinuation was ‘flare’ in SB4 patients (12%, 4 of 33 AEs), and ‘skin reactions at the injection site’ in oETN patients (26%, 18 of 68 AEs). Overall, injection site reactions occurred in 7% (n=23) of oETN patients and 3% (n=7) of SB4 patients (leading to discontinuation in only one patient).
Conclusion: We found higher retention rates for bionaive patients starting the biosimilar SB4 compared to those starting the originator oETN. Whereas both treatments were equally effective, patients treated with oETN had more injection site reactions. We cannot rule out selection bias since there is practice variation in the usage of biosimilars in Germany (regional quota systems). In addition, patients receiving either oETN or SB4 were not entirely comparable (e.g. more comorbidi-ties on oETN).
To cite this abstract in AMA style:
Baganz L, Meißner Y, Herzer P, Braun J, Gräßler A, Strangfeld A, Zink A. Treatment Continuation on the Etanercept Original in Comparison with a Biosimilar [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/treatment-continuation-on-the-etanercept-original-in-comparison-with-a-biosimilar/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-continuation-on-the-etanercept-original-in-comparison-with-a-biosimilar/