Session Information
Date: Monday, October 22, 2018
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Of the very few studies describing Down syndrome arthropathy (DA), crude prevalence estimates indicate DA maybe as common as juvenile idiopathic arthritis (JIA), however, DA is still largely under recognized at onset. Previous studies indicate the majority of DA present with greater than 5 affected joints and bony changes at diagnosis. Additionally, treatment is complex and most require a second-line therapy after Methotrexate (MTX) due an increased methotrexate toxicity. Further, gaps in literature exist around optimal treatment approach, escalation and response. The objective of this study was to investigate treatment and response of DA.
Methods: In a retrospective chart review that took place at two tertiary care hospitals, potential DA patients were identified through electronic medical record system (EMR) from January 1, 1995 to December 31, 2015. ICD-9-CM codes were used to identify patients (less than 18 years of age) with both Down Syndrome (DS; 758.0) and JIA (714.3, 714.31, 714.32, 714.33). Individual charts were then manually reviewed to confirm diagnosis of DS and JIA. Chart review included analysis of all documents included in EMR, including demographic data, clinical visits, imaging studies and laboratory results.
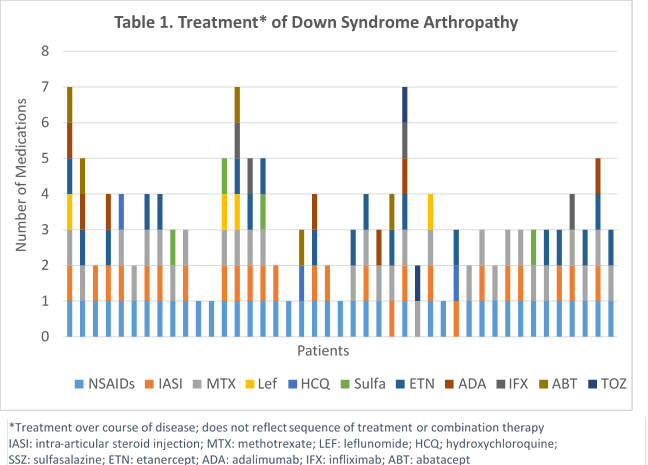
Results: Fourty-three patients met inclusion criteria with a mean follow-up period of 6 years (SD 4.4). Patients had a mean 7.4 year (SD 3.9) age at onset of musculoskeletal symptoms with a mean 19 months (SD 17) to JIA diagnosis. Patients were mostly female (58%). At JIA diagnosis 63% had a polyarticular, RF negative presentation, 70% reported morning stiffness with an average of 15 active joints (SD 13), 14 limited joints (SD 13), and mean physician global of disease activity 4.5 (SD 2.2). Most patients (93%) were started on nonsteroidal anti-inflammatory drugs (NSAIDs) at diagnosis with 28% simultaneously starting a disease modifying antirheumatic drug (DMARD), and 5% a Biologic. Over the course of disease 74% used a DMARD (94% MTX) and 47% used a biologic (83% Enbrel). Eight patients (25%) had at least one change in DMARD and nine patients (39%) had at least one change in biologic therapy (Table 1.). At the last visit there were significantly (p < 0.001) less active (3 [SD 5]) and limited joints (5 [SD 9]), and the mean physician global of disease activity was 1.3 (SD 1.5). Of those on DMARD therapy 60% were discontinued due to side effects and 39% had inadequate response to first-line biologic therapy.
Conclusion: Down syndrome arthropathy remains under recognized, but does have a significant reduction in active and limited joints when treated with NSAIDs, DMARDs, and biologics, however, treatment approach, optimal therapy and escalation is unclear. Other barriers that inhibit optimal treatment and response are DMARD toxicity and anti-TNF effectiveness. More research is currently needed to determine optimal therapy approach.
To cite this abstract in AMA style:
Jones JT, Talib N, Lovell DJ, Becker ML. Treatment and Response of Down Syndrome Arthropathy [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/treatment-and-response-of-down-syndrome-arthropathy/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-and-response-of-down-syndrome-arthropathy/