Session Information
Date: Wednesday, November 16, 2016
Title: Rheumatoid Arthritis – Clinical Aspects VII: The Impact of Treating to Target
Session Type: ACR Concurrent Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose:
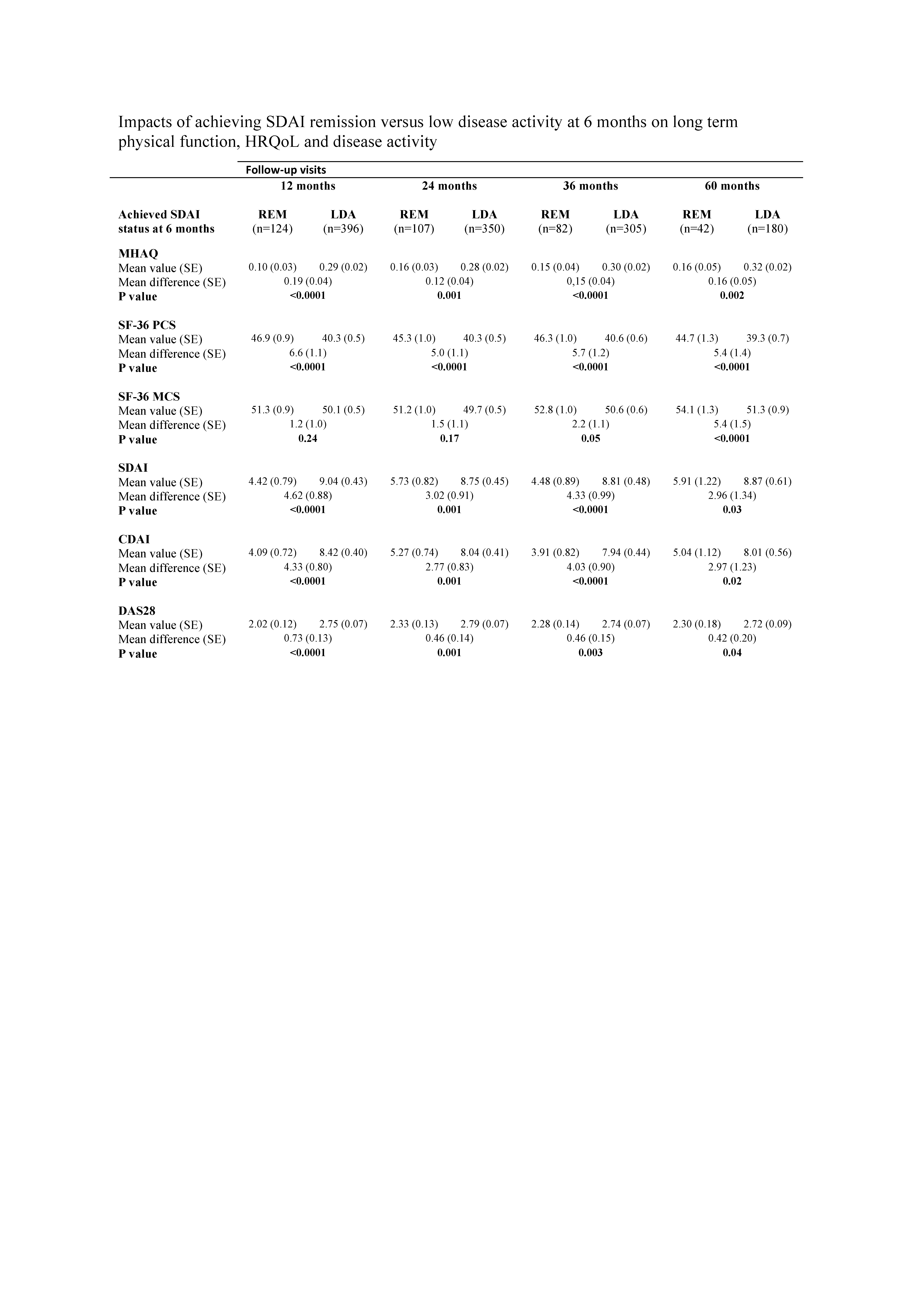
The management of rheumatoid arthritis (RA) has evolved considerably during the last couple of decades, with current recommended practice being a treat-to-target approach, involving early intervention and tight monitoring of treatment effect. When starting a new therapy, the recommended target is remission (REM) or alternatively low disease activity (LDA). Limited data exist on the impacts of reaching REM rather than LDA on long-term outcomes. The objective of this study was to compare RA-patients who achieved Simplified Disease Activity Index (SDAI) REM versus LDA 6 months after initiating disease-modifying anti-rheumatic drug (DMARD) therapy, with regard to physical function, Health Related Quality of Life (HRQoL) and disease activity during 5-year follow-up in a real-life clinical setting.Methods:
Data were provided by the Norwegian DMARD study (NOR-DMARD), a multicentre longitudinal observational study. We selected DMARD-naive patients with RA who were consecutively enrolled from December 2000 to April 2009 (n=1617), starting treatment with methotrexate (n=1197), other conventional synthetic DMARDs (n=380) or a biological DMARD (n=33). Data on each patient were collected at baseline, after 3, 6 and 12 months, and yearly thereafter, including the modified Health Assessment Questionnaire (MHAQ), the Medical Outcomes Study 36-item Short-Form Health Survey (SF-36) with Physical and Mental Components Summary scores (PCS and MCS, respectively) and assessments that allowed the calculation of the composite disease activity scores SDAI, Clinical Disease Activity Index (CDAI) and the Disease Activity Score based on 28 joint counts with ESR (DAS28). Multivariate linear mixed models were used to explore the effect of SDAI status at 6 months on physical function (MHAQ), HRQoL (SF-36 PCS and MCS) and disease activity (SDAI, CDAI, DAS28) during 1-5 year follow-up. The statistical models were adjusted for potential baseline confounders such as age, sex, disease duration and disease activity.Results:
Of the 1617 eligible patients at baseline, 1301 (80.5%) had a registered visit at 6 months. 602 patients were in either SDAI REM (n=143, 11.0%) or LDA (n=459, 35.3%) at 6 months and were included in the main analyses. The achievement of SDAI REM rather than SDAI LDA at 6 months was associated with significantly better physical function (MHAQ) and HRQoL (SF-36 PCS) and with lower disease activity (SDAI, CDAI, DAS28) at all yearly follow-up visits between 1 and 5 years (table).Conclusion:
The achievement of SDAI REM rather than LDA 6 months after initiating DMARD-therapy, lead to better long term functional outcomes during 5-year follow-up in a real-life clinical setting. The results support the use of a stringent treatment target, such as SDAI REM, when initiating DMARD-therapy in RA-patients.
Disclosure: V. Norvang, None; E. Lie, None; I. C. Olsen, None; E. K. Kristianslund, None; T. K. Kvien, Biogen, BMS, Boehringer Ingelheim, Celltrion, Eli Lilly, Epirus, Hospira, Merck-Serono, Novartis, Orion Pharma, Prizer, Sandoz, UCB, 5; T. Uhlig, None.
To cite this abstract in AMA style:
Norvang V, Lie E, Olsen IC, Kristianslund EK, Kvien TK, Uhlig T. Treat-to-Target in RA: Does Early Simplified Disease Activity Index (SDAI) Remission Lead to Better 5-Year Functional Outcomes Than SDAI Low Disease Activity? [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/treat-to-target-in-ra-does-early-simplified-disease-activity-index-sdai-remission-lead-to-better-5-year-functional-outcomes-than-sdai-low-disease-activity/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treat-to-target-in-ra-does-early-simplified-disease-activity-index-sdai-remission-lead-to-better-5-year-functional-outcomes-than-sdai-low-disease-activity/