Session Information
Date: Sunday, November 13, 2022
Title: Systemic Sclerosis and Related Disorders – Basic Science Poster
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Systemic Sclerosis (SSc) is an autoimmune connective tissue disease characterized by immune system activation, endothelial dysfunction and widespread tissue fibrosis. Activation of the monocyte/macrophage system in SSc is thought to contribute to SSc pathogenesis via fibroblast activation. However, little is known about the potential contribution of fibroblasts to the phenotype of tissue-resident macrophages in SSc. To address this gap, we performed a microarray analysis on macrophages cultured with conditioned media (CM) from SSc and healthy control fibroblasts.
Methods: Monocytes were isolated using CD14 magnetic beads from healthy PBMCs and differentiated to macrophages in the presence of MCSF. Macrophages were then incubated for 24 hours with CM from an equal number of dermal fibroblasts from 4 different SSc subjects (SS) and 4 different healthy subjects (HS). Unstimulated macrophages (UMø) were used as control. Microarray analysis was performed using the Affymetrix GeneChip™human 2.0 ST gene array. Analysis for differentially regulated genes was conducted and Ingenuity pathway analysis was used to identify differentially regulated canonical pathways.
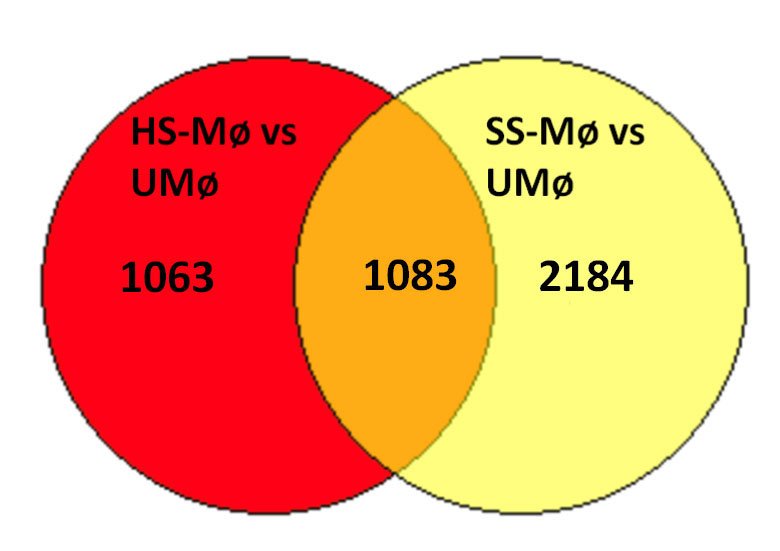
Results: Compared to baseline, unstimulated macrophages (UMø) a total of 2146 and 3267 genes were identified as significantly differentially expressed (p< 0.05) in macrophages exposed to CM from HS fibroblasts (HS-Mø) and SS fibroblasts (SS-Mø) respectively, with only 1083 genes overlapping between the two (Figure 1). Compared to HS, exposure to CM from SS induced changes in several different pathways, including angiogenesis inhibition via thrombospondin 1 (TSP1), apoptosis activation and pyroptosis induction (Figure 2). TSP1, a matricellular protein with anti-angiogenetic, profibrotic and proinflammatory properties, secreted by fibroblasts and macrophages and increased in SSc, was the most significantly upregulated anti-angiogenesis gene. Related to activation of pyroptosis, we found significantly increased expression of gasdermin-B, GBP1, CASP3, and several toll-like receptors (TLR1, TLR2, TLR8). Importantly, gasdermin-mediated macrophage pyroptosis has been recently implicated in the pathogenesis of fibrosis in SSc.
Conclusion: SSc fibroblasts have a distinctive effect on macrophages, inducing activation of canonical pathways related to apoptosis, pyroptosis, and inhibition of angiogenesis via TSP1. By releasing TSP1 in response to SSc fibroblasts, macrophages may limit angiogenic responses, which are critical to repair, to favor fibrosis progression. Our results suggest that a complex cascade of feedback regulation between fibroblasts, macrophages, and endothelial cells, may contribute to SSc pathogenesis.
To cite this abstract in AMA style:
Zertuche J, Dinc T, El Adili F, Ligresti G, Bujor A. Transcription Analysis of Macrophages Reveals Important Changes Induced by Systemic Sclerosis Fibroblasts [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/transcription-analysis-of-macrophages-reveals-important-changes-induced-by-systemic-sclerosis-fibroblasts/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/transcription-analysis-of-macrophages-reveals-important-changes-induced-by-systemic-sclerosis-fibroblasts/