Session Information
Date: Wednesday, November 15, 2023
Title: Abstracts: Systemic Sclerosis & Related Disorders III: Clinical Trials
Session Type: Abstract Session
Session Time: 11:00AM-12:30PM
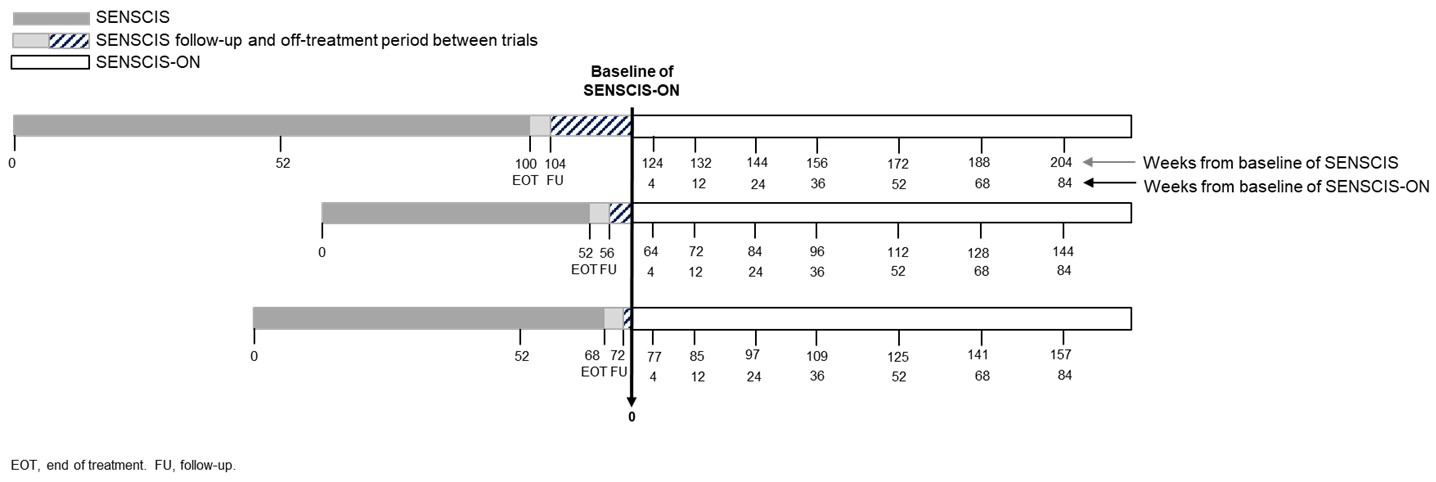
Background/Purpose: The SENSCIS trial enrolled patients with SSc-ILD without a requirement for them to have evidence of recent progression. During the trial, nintedanib reduced the rate of decline in FVC (mL/year) versus placebo. The open-label extension of the SENSCIS trial, SENSCIS-ON, assessed changes in FVC in patients treated with nintedanib over the longer term. We assessed the trajectory of FVC decline in patients who received placebo in the SENSCIS trial and then nintedanib in SENSCIS-ON.
Methods: In the SENSCIS trial, patients with SSc-ILD were randomized to receive nintedanib or placebo double-blind until the last patient reached week 52 but for ≤100 weeks. Patients who completed the SENSCIS trial on treatment (nintedanib or placebo) and attended a follow-up visit were eligible to enter SENSCIS-ON. The protocol allowed an off-treatment period between SENSCIS and SENSCIS-ON of ≤12 weeks. We calculated the trajectory of FVC in patients who received placebo in SENSCIS and initiated nintedanib in SENSCIS-ON. The baseline measurement in SENSCIS-ON was considered the anchor measurement (time point 0) (Figure 1). FVC was measured at time points before the anchor measurement (when patients were receiving placebo in SENSCIS or were off treatment between SENSCIS and SENSCIS-ON) and at time points after the anchor measurement (when patients were receiving open-label nintedanib in SENSCIS-ON). Analyses were descriptive and based on observed FVC values.
Results: In total, 231 patients received placebo in SENSCIS and then nintedanib in SENSCIS-ON. In these patients, mean (SD) FVC was 2593 (833) mL at baseline of SENSCIS and 2441 (833) mL at baseline of SENSCIS-ON. The mean decline in FVC in the 52 weeks prior to baseline of SENSCIS-ON (when patients were receiving placebo in SENSCIS or were off treatment) was −98.5 mL (Figure 2). The mean decline in FVC from baseline of SENSCIS-ON to week 52 of SENSCIS-ON (when patients were receiving nintedanib) was −42.8 mL (Figure 2).
Conclusion: In patients who received placebo in the SENSCIS trial, the decline in FVC over 52 weeks was reduced by approximately 57% following initiation of open-label nintedanib in SENSCIS-ON. These analyses illustrate the progressive nature of SSc-ILD in the population enrolled in SENSCIS and the effectiveness of nintedanib on slowing the progression of SSc-ILD.
To cite this abstract in AMA style:
Distler O, Vonk M, Azuma A, Mayes M, Khanna D, Highland K, Toenges G, Alves M, Allanore Y. Trajectories of Forced Vital Capacity (FVC) in Patients with Systemic Sclerosis-Associated Interstitial Lung Disease (SSc-ILD) [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/trajectories-of-forced-vital-capacity-fvc-in-patients-with-systemic-sclerosis-associated-interstitial-lung-disease-ssc-ild/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/trajectories-of-forced-vital-capacity-fvc-in-patients-with-systemic-sclerosis-associated-interstitial-lung-disease-ssc-ild/