Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: The efficacy of belimumab in treating SLE has been demonstrated in several phase 3 randomized clinical trials (RCTs). These trials showed belimumab efficacy on clinical parameters and patient-reported outcomes (PROs), including fatigue assessed by the Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-F) questionnaire. However, there was a considerable individual variation regarding measures of disease activity and fatigue over time, with only mild associations between those and clinical outcomes. Identifying patient subgroups with similar characteristics, as assessed by clinical and psychometric measures, could enhance our understanding of SLE progression, improving statistical power and generalizability in clinical trial design.
Methods: A post-hoc analysis of data from three phase 3 RCTs of belimumab in SLE was conducted (BLISS-52, BLISS-76, BLISS-SC).Patients with at least two visits from baseline, complete BILAG, SLEDAI-2K, PGA, FACIT-F score, and steroid use data were included. Growth mixture modeling via the lcmm package in R identified latent classes. Data were modeled with the multlcmm function to determine latent classes, assuming a common underlying process for all outcomes. Four-week trends of BILAG, SLEDAI, FACIT-F (range: 0-52, the lower the better), PGA (range: 0-3), and the proportion of patients achieving LLDAS or DORIS remission were plotted in relation to latent classes.
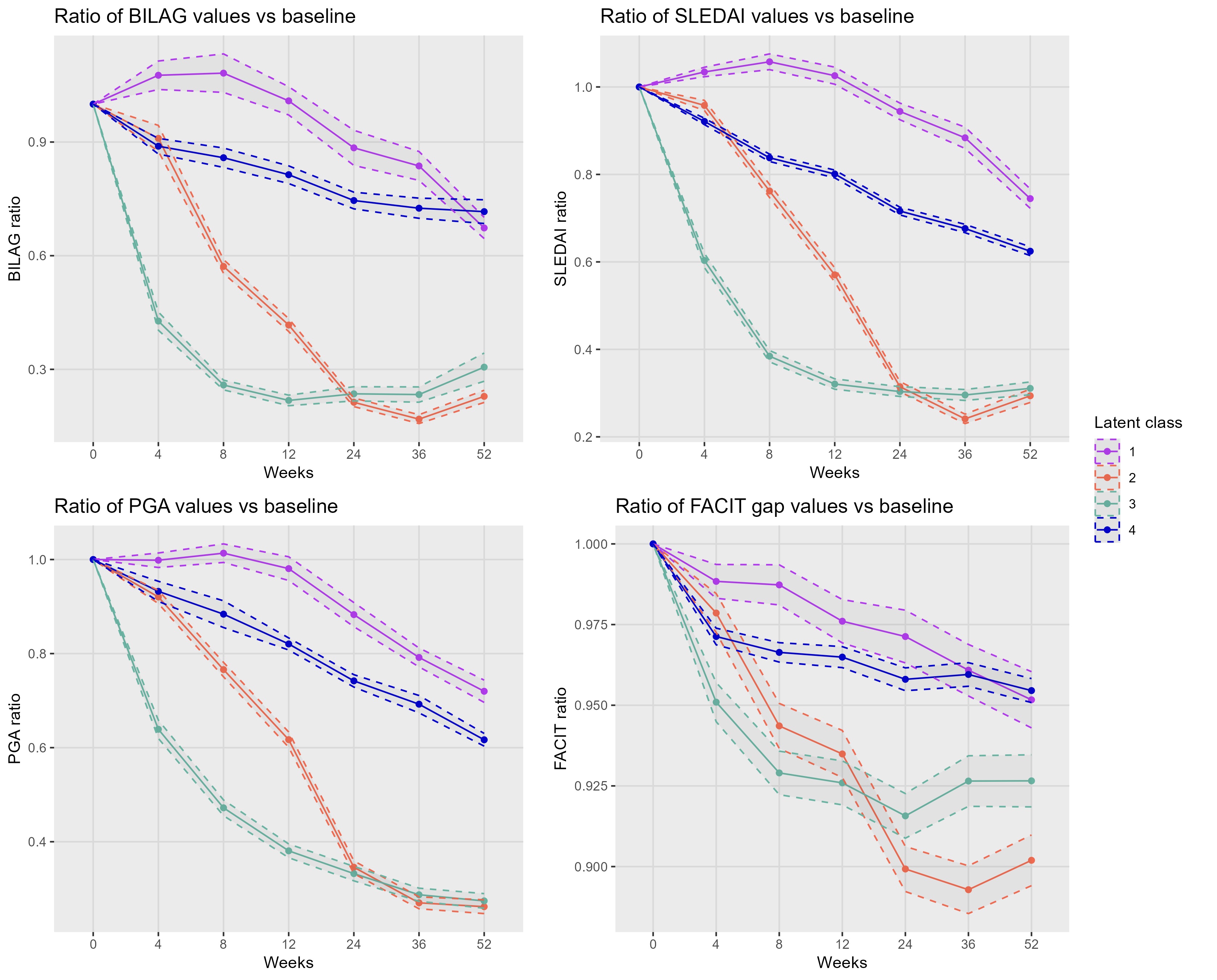
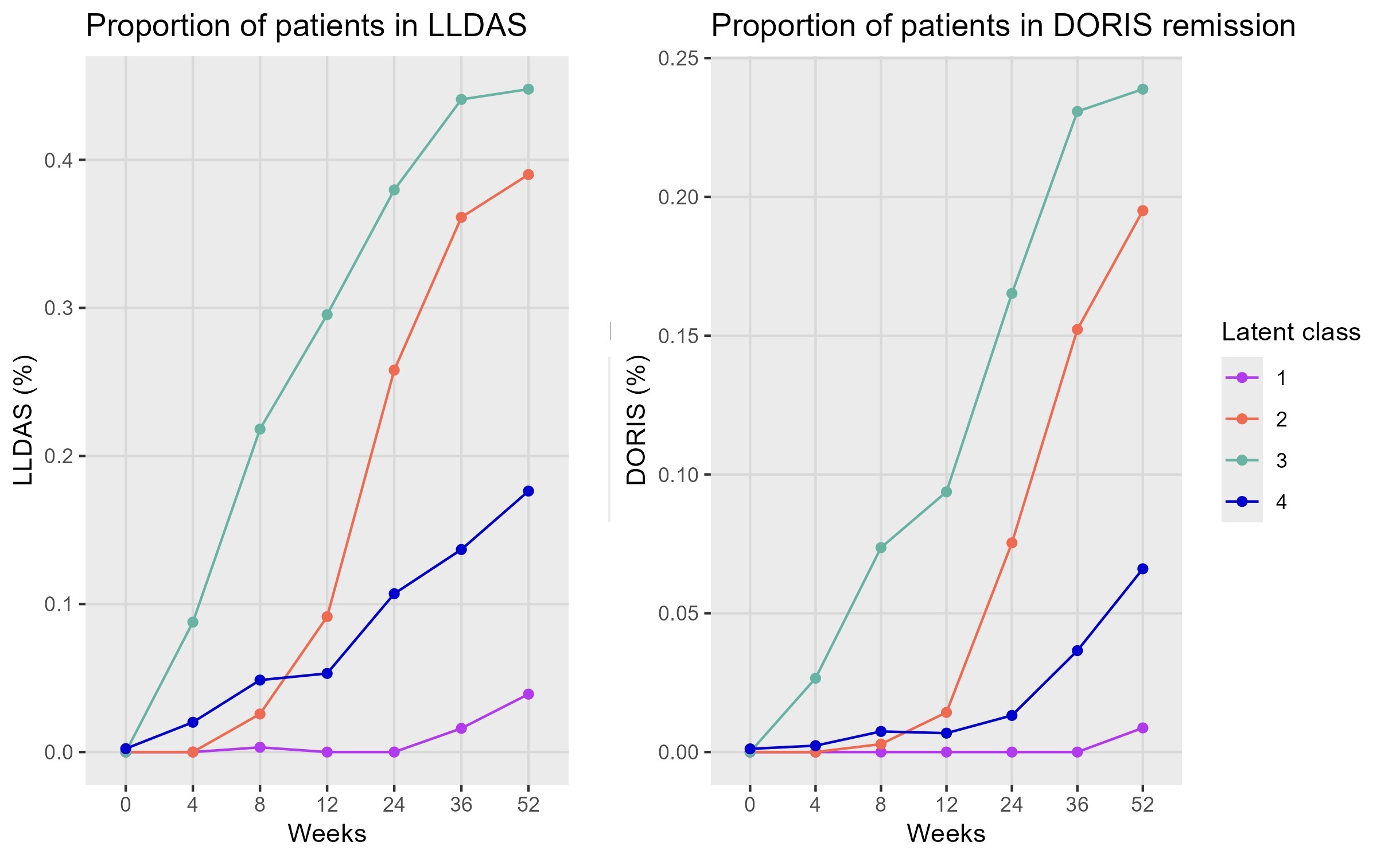
Results: A total of 2868 patients (94.6% female) were analyzed. Mean (± standard deviation) baseline disease duration was 6.6±6.6 years, SDI scores 0.7±2, BILAG scores 17±7.8, SLEDAI-2K 10.2±3.6, PGA 1.5±0.5, daily prednisone dose 11±8.9 mg, and FACIT-F scores 30.6±11.9. In the initial model, steroids had high standard errors, indicating a weak link with the latent process and that they do not share much information with the other variables. A refined model, considering only clinical measures and FACIT-F, corrected for treatment and SDI scores was then bult (no other covariates improved the fit). Four classes (quick responders, slow responders, moderate-disease non-responders, and severe-disease non-responders) best described SLE progression (Figure 1). The proportion of patients achieving LLDAS or DORIS remission correlated with latent classes (Figure 2). Baseline characteristics alone or in combination could not predict disease progression.
Conclusion: SLE progression can be described by four distinct trajectories linking PROs and disease severity measures. This classification may personalize treatment plans and enhance clinical trial design by identifying distinct patient subgroups. Furthermore, this post-hoc classification could be valuable for biological studies aimed at differentiating the characteristics of the diverse trajectories, given that no clinical variable alone can predict them.
To cite this abstract in AMA style:
Parodis I, Lindblom J, Tsoi A, Nikolopoulos D, Beretta L. Trajectories of Disease Evolution upon Treatment Initiation in Systemic Lupus Erythematosus: Pooled Results from Three Randomized Clinical Trials of Belimumab [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/trajectories-of-disease-evolution-upon-treatment-initiation-in-systemic-lupus-erythematosus-pooled-results-from-three-randomized-clinical-trials-of-belimumab/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/trajectories-of-disease-evolution-upon-treatment-initiation-in-systemic-lupus-erythematosus-pooled-results-from-three-randomized-clinical-trials-of-belimumab/