Session Information
Date: Sunday, November 8, 2020
Title: Pediatric Rheumatology – Clinical Poster II: Systemic JIA, Autoinflammatory, & Scleroderma
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Macrophage Activation Syndrome (MAS) and secondary Hemophagocytic Lymphohistiocytosis (sHLH) are hyperinflammatory conditions caused by a cytokine storm, in which IFNγ plays a pivotal role. In this study we aimed to evaluate clinical characteristics of sHLH, MAS and systemic Juvenile Idiopathic Arthritis (sJIA) patients at disease onset. We compared laboratory parameters of hyperinflammation (platelet count, ferritin, AST, triglycerides, fibrinogen) and IFNγ related biomarkers in samples collected in three different time points: active disease (T0), 7-10 days from starting therapy (T1) and in clinical inactive disease (from 1 to 3 months from onset) (T2).
Methods: Routine laboratory parameters of disease activity and severity were collected from a cohort of 82 patients with sHLH (38), MAS in the context of sJIA (26), and sJIA (18) at T0, T1 and T2. Serum levels of the IFNγ related biomarkers (CXCL9, CXCL10, neopterin and IL-18) were measured at each time points by ELISA.
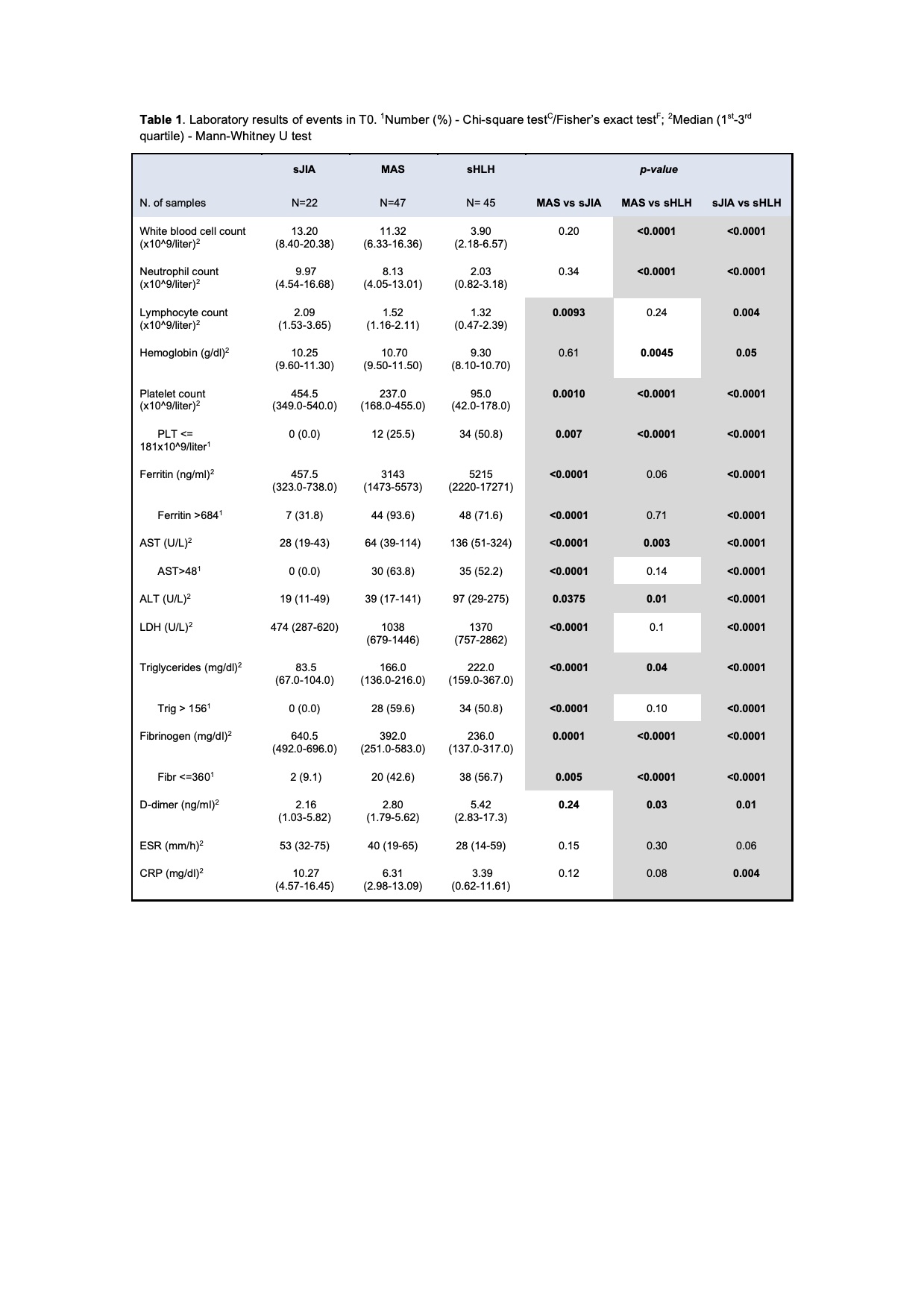
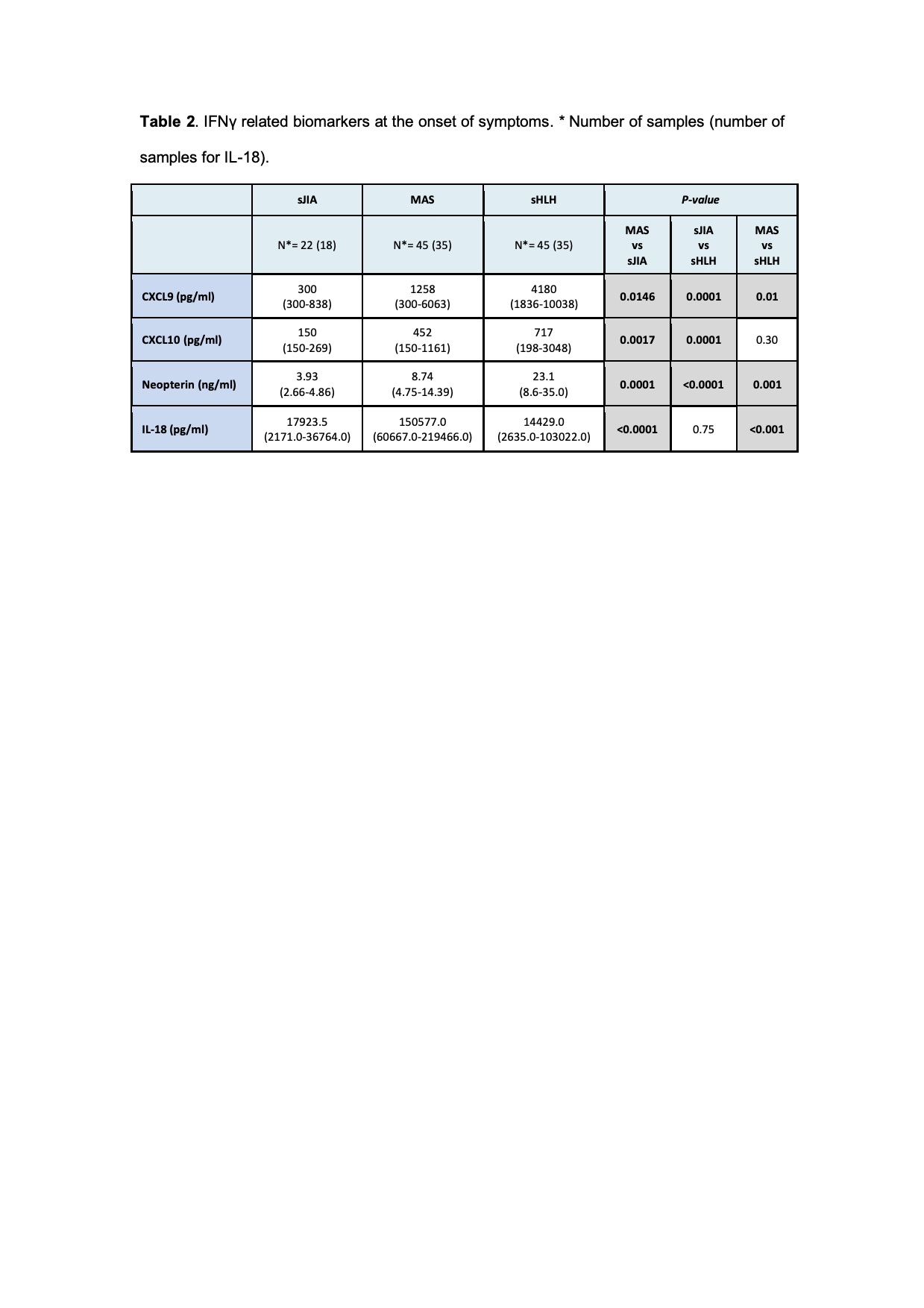
Results: A total of 306 samples were collected. Fever was present in the majority of patients (95%), while splenomegaly was more frequently in MAS (65%) and sHLH (63%) compared to sJIA (17%). Laboratory characteristics at T0 are detailed in Table 1. Using the 2016 classification criteria for MAS [1], we found that platelet count is a specific parameter, any patient with sJIA had a value < 181x109/liter; while ferritin is a sensitive one, 94% of patients with MAS had ferritin > 684 mg/ml. CXCL9, CXCL10 and neopterin levels in T0 were significantly higher in MAS and in sHLH compared to sJIA, while IL-18 was significantly higher only in MAS group (Table 2). The ROC curves performed for each biomarker in MAS showed a statistically significant AUCs (p< 0.05). In sHLH the AUCs was significant for CXCL9, CXCL10 and Neopterin (p< 0.0001), while for IL-18 was not (p= 0.9) (Figure 1). In MAS CXCL9 and neopterin were significantly correlated to laboratory parameters of hyperinflammation as well as IL-18, except for ferritin. In sHLH only neopterin was significantly correlated to platelet count and triglycerides. CXCL9, CXCL10, neopterin and IL-18 levels progressively lowered at T1 and normalized in T2. CXCL9 decreased faster compared to neopterin, with a similar trend of laboratory parameters.
Conclusion: Our results confirme that platelet counts and ferritin have and high specificity and sensitivity, respectively, to diagnose MAS in the context of sJIA. Moreover, our results confirmed that IFNγ related biomarkers are significantly high in patients with MAS and sHLH and could be useful for diagnosis in addition to traditional laboratory parameters. CXCL9 seems to be more related to active disease, while neopterin seems to be more useful to follow the disease course. As already known, IL-18 is a specific biomarker for MAS.
Reference.
1. Ravelli A et al. Ann Rheum Dis. 2016 Mar;75(3):481-9.
To cite this abstract in AMA style:
De Matteis A, Pires Marafon D, Caiello I, Pardeo M, Marucci G, Sacco E, Prencipe G, De Benedetti F, Bracaglia C. Traditional Laboratory Parameters and New Biomarkers in Macrophage Activation Syndrome and Secondary Hemophagocytic Lymphohistiocytosis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/traditional-laboratory-parameters-and-new-biomarkers-in-macrophage-activation-syndrome-and-secondary-hemophagocytic-lymphohistiocytosis-2/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/traditional-laboratory-parameters-and-new-biomarkers-in-macrophage-activation-syndrome-and-secondary-hemophagocytic-lymphohistiocytosis-2/