Session Information
Date: Monday, November 14, 2022
Title: SLE – Diagnosis, Manifestations, and Outcomes Poster III: Outcomes
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Most late-phase randomised controlled trials (RCTs) of novel drugs for systemic lupus erythematosus (SLE) have failed to meet their primary endpoint or shown contradictory efficacy results(1). While many factors contribute to RCT failure, how best to measure treatment benefit remains a challenge(1). For product approval, regulators require evidence of clinical benefit, defined as a positive effect on how patients “feel, function or survive”(2). Current endpoint assessments are based on legacy disease activity measures not designed for use in RCTs or pursuant to current guidelines, and lacking patient input in their development. They are further limited by use of binary variables, glossary-defined thresholds and weighting, and inclusion of concepts not proven to reflect meaningful health aspects for SLE patients. We report the formation and initial outcomes of a global academic-industry partnership to develop a novel, patient-centred, clinician-reported outcome measure (ClinRO) for SLE RCTs: Treatment Response Measure for SLE (TRM-SLE).
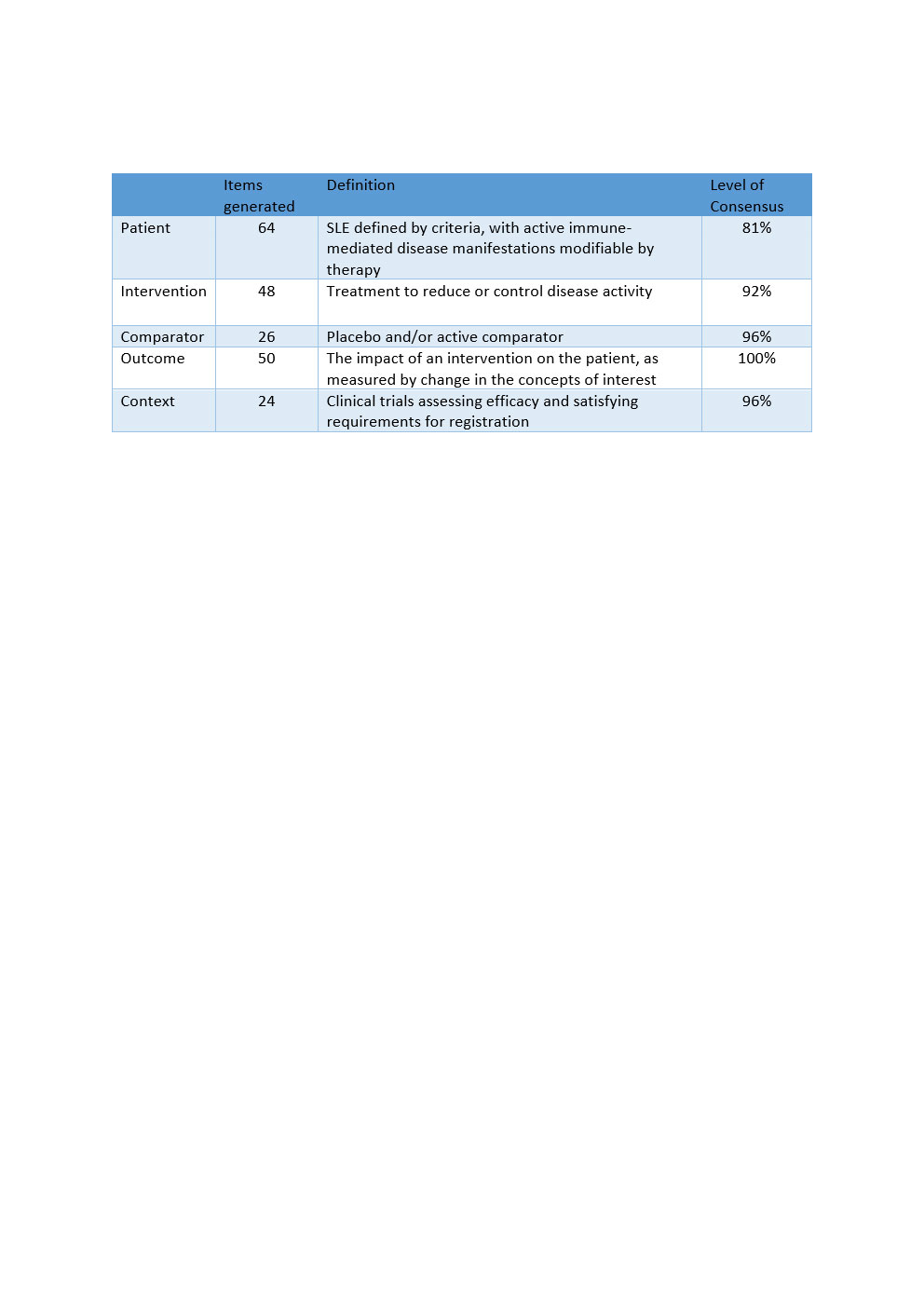
Methods: A global panel of clinical-academic, metrology and regulatory experts, patient representatives and experts from 10 companies developing SLE drugs, was convened. Using consensus and participatory methods, we generated a conceptual definition for the setting, manner and purpose of TRM-SLE, with reference to regulatory guidance and using the PICO-C (Patient; Intervention; Comparator; Outcome; Context) framework. Literature review, item generation and grouping, moderated discussion and real-time voting via virtual platforms were used, with a predefined threshold of 70% agreement set to establish consensus.
Results: 45 experts from North and Latin America, Europe, and the AsiaPacific participated. 212 items were generated, including items for Patient (64), Intervention (48), Comparator (26), Outcome (50), and Context (24). Via moderated discussion and multiple rounds of voting, disagreements, which tended to be over semantic rather than definition or scope disparities, were resolved. The conceptual definition for TRM-SLE was defined with high agreement (81-100%, Table 1).
A protocol has been designed for subsequent steps that will use structured methods involving clinician researchers, industry, regulatory and metrology experts, SLE patients, and patient organisation representatives. These steps will determine the concepts of interest and measurements to be included in TRM-SLE, according to its defined context of use. Construct validity and measurement properties will be established before integration in an SLE RCT endpoint for regulatory approval worldwide.
Conclusion: A global academic-industry partnership has completed the first steps in development of an SLE ClinRO that, by design, is a measure of treatment response rather than disease activity and conforms to modern best practice and regulatory guidance.
References
1. Dolgin E. Lupus in crisis: as failures pile up, clinicians call for new tools. Nat Biotechnol. 2019;37(1):7-8.
2. Powers, J. H. et al. Clinician-Reported Outcome Assessments of Treatment Benefit: Report of the ISPOR Clinical Outcome Assessment Emerging Good Practices Task Force. Value Health 2017; 20:2–14.
To cite this abstract in AMA style:
Connelly K, Eades L, Koelmeyer R, Ayton D, Golder V, Kandane-Rathnayake R, Gregory-Wong K, Al-Mossawi H, Andersen J, Aranow C, Arnaud L, Askanase A, Banerjee S, Barbey C, Brunner H, Buie J, Burke L, Cornet A, Costenbader K, Dall'Era M, Dantata K, Delev N, Eldred A, Friedman A, Furie R, Garces S, Grasela D, Guay H, Guenther O, Juarez M, Kafka S, Kalunian K, Karis E, Lahoud Y, Lindholm C, Lockman J, Lupton C, Maller J, Marion A, Marquis P, Merrill J, Morel T, Mosca M, Pincus Y, Pomponi S, Pons-Estel G, Ross Terres J, Sibley C, Silk M, Roy S, Simon L, Sorrentino A, Stach C, Stojan G, Sun Y, Tanaka Y, Thomas E, van Vollenhoven R, Vazquez Mateo C, Vital E, Werth V, Zollars E, Zuraw Q, Morand E. Towards a Novel Clinician-Reported Outcome Measure for SLE – Outcomes of an International Consensus Process [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/towards-a-novel-clinician-reported-outcome-measure-for-sle-outcomes-of-an-international-consensus-process/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/towards-a-novel-clinician-reported-outcome-measure-for-sle-outcomes-of-an-international-consensus-process/