Session Information
Date: Sunday, November 13, 2022
Title: Vasculitis – Non-ANCA-Associated and Related Disorders Poster II
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: SPI-62 is a potent 11b-hydroxysteroid dehydrogenase type 1 (HSD-1) inhibitor entering Phase 2 development as adjunctive therapy to prednisolone in polymyalgia rheumatica, as well as for treatment of Cushing’s syndrome and autonomous cortisol secretion. HSD-1, an intracellular enzyme, activates glucocorticoids in target tissues in which glucocorticoid medicines are associated with morbidity including liver, adipose, muscle, and skin. In Phase 1 clinical trials SPI-62 was generally well tolerated and associated with maximal liver and brain HSD-1 inhibition. Our objective was to demonstrate mitigation by SPI-62 of corticosterone (CORT) adverse effects in mouse.
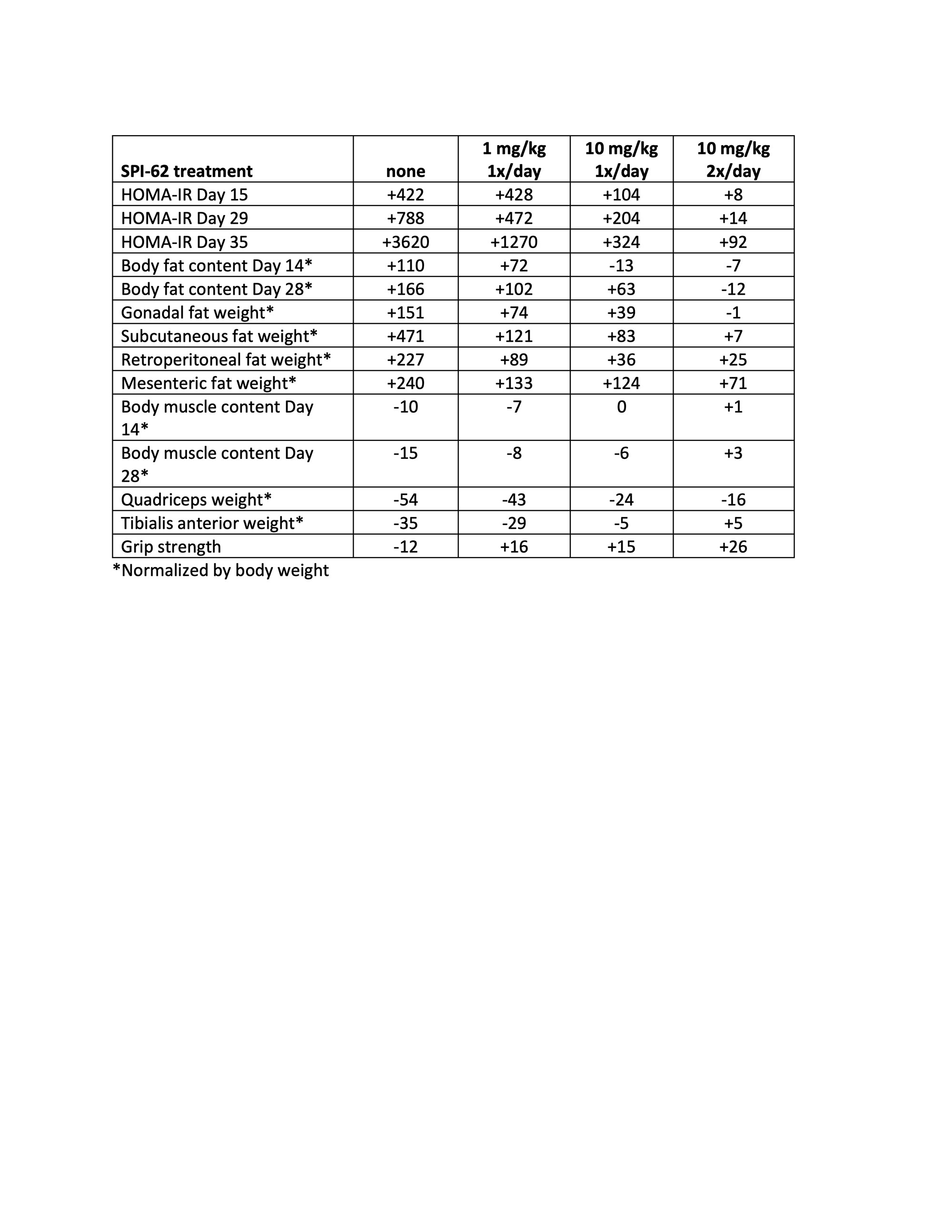
Methods: C57BL/6 male mice (age 7 weeks; n=14 per group) were administered CORT (100 mg/mL in drinking water) and SPI-62 (by gavage in 0.5% HPMC; 0, 1, or 10 mg/kg/day or 10 mg/kg twice daily) for 35 days. A control group received no CORT or SPI-62. Body weight was assessed daily and food consumption twice weekly. Whole body muscle and fat amounts were measured at Days 0, 14, and 28 using an EchoMRI-130H body composition analyzer. Blood samples for fasting glucose and insulin were obtained at Days 1 (pre-dose), 15, 29, and 35. An open field test was conducted on Day 22. A grip strength test was performed on Day 28. After sacrifice on Day 36, gonadal, subcutaneous, retroperitoneal, and mesenteric fat, quadriceps, and tibialis anterior were dissected and weighed, and skin was formalin fixed and paraffin embedded.
Results: One control mouse died due to accidental gavage injury. Two mice who received CORT + 0.5% HPMC died on Days 11 and 19. CORT resulted in increased food consumption which was normalized by SPI-62 in a dose-dependent manner. CORT-treated mice showed reduced body weight gain for 2 weeks then accelerated body weight gain. SPI-62 prevented body weight gain acceleration in a dose-dependent manner. CORT effects on dermal thickness and structure were less prominent in mice who also received SPI-62. No effect of CORT or SPI-62 was observed in the open field test. SPI-62 prevented CORT adverse effects of insulin resistance, increased adiposity, skeletal myoatrophy, and grip strength reduction (Table).
Conclusion: SPI-62 prevented several CORT adverse effects in mouse, demonstrating that blockade of local intracellular glucocorticoid activation by a HSD-1 inhibitor in target tissues can mitigate glucocorticoid toxicity. These results suggest that SPI-62 has potential to similarly mitigate adverse effects of glucocorticoid medications in human. A placebo-controlled Phase 2 clinical trial in patients with polymyalgia rheumatica has been initiated to compare prednisolone effects with and without SPI-62.
To cite this abstract in AMA style:
Pan X, Hou Q, Xu J, Ma Y, Li J, Li M, Su J, Shi X, Bracken W, Katz D. Toward Safer Glucocorticoid Therapy [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/toward-safer-glucocorticoid-therapy/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/toward-safer-glucocorticoid-therapy/