Session Information
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Tanezumab (TNZ) is an antibody against nerve growth factor that has demonstrated efficacy in the management of osteoarthritis (OA). Due to the potential risk of rapidly progressive OA (RPOA), recent phase 3 studies of subcutaneous (SC) TNZ have included a comprehensive prospective assessment of joint safety. This analysis summarizes data on patients (pts) who underwent total joint replacement (TJR) in the recent phase 3 OA TNZ studies.
Methods: In NCT02697773, pts with OA received placebo (PBO), TNZ 2.5 mg or 2.5 mg then 5 mg (TNZ 2.5/5 mg) for 16 weeks. In NCT02709486, pts received PBO or TNZ 2.5 or 5 mg for 24 weeks. In NCT02528188, pts received TNZ 2.5 or 5 mg or NSAIDs for 56 weeks. TNZ and matching PBO were given SC every 8 weeks; NSAID and matching PBO were taken orally twice daily. All studies had a 24-week safety follow-up period. Subgroup analyses were performed to explore potential associations between the incidence of TJR and baseline (BL) demographic/clinical characteristics or post-BL outcomes. In all studies, TJRs were adjudicated by a blinded external Adjudication Committee to determine any association with joint safety events including RPOA type 1, RPOA type 2, subchondral insufficiency fracture (SIF), primary osteonecrosis, or pathological fracture. For each pt, the most painful hip or knee joint with radiographic OA was selected as the index joint. Pain was assessed in all joints but efficacy assessments were only for the index joint. Pts could also have had OA in non-index joints.
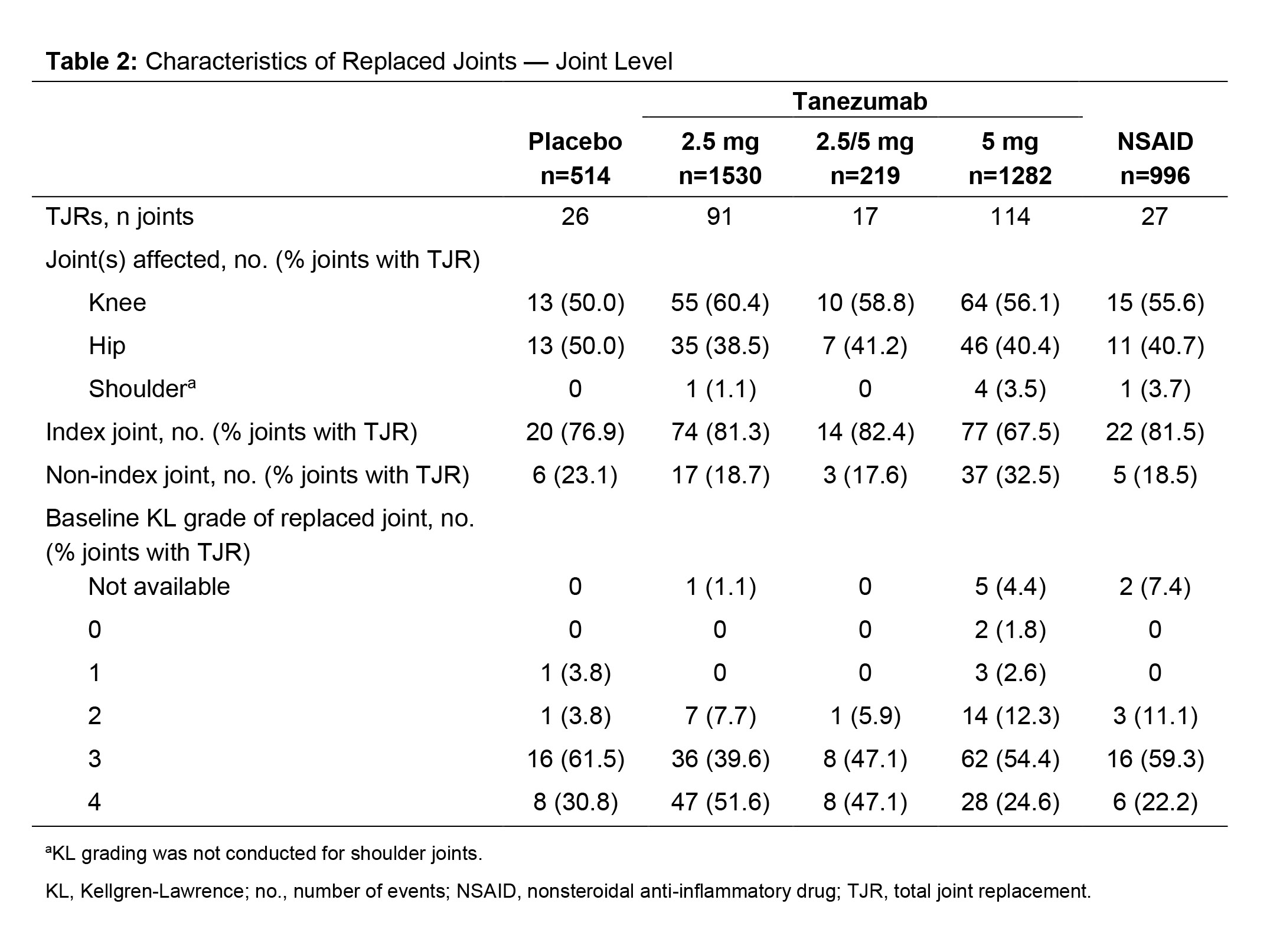
Results: Across the 3 studies (N=4541 pts), 248 pts had ≥1 TJR (26 ≥2 TJRs) with an incidence of 2.6–7.8% across groups (Table 1). In all, 83% of pts with a TJR had surgery on their index joint, the proportion of which was similar in the PBO, TNZ 2.5 mg, and NSAID groups (84.6–88.1%), lower in the TNZ 5 mg (77.0%) group, and higher in the TNZ 2.5/5 mg (93.3%) group (Table 1). Most TJRs occurred in joints with Kellgren-Lawrence (KL) grade 3 or 4 at BL (Table 2). In the TNZ 5 mg group, 2 pts had TJR of a joint that was KL grade 0 at BL: 1 was adjudicated to be associated with RPOA2 and 1 as a post traumatic subchondral fracture following an accidental fall. The majority (73.3–81.0%) of pts across treatment groups had no prior history of TJR. Of the 275 TJRs reported across groups, 50.0–60.4% occurred in a knee, 38.5–50.0% in a hip and 0–3.7% in a shoulder (Table 2). Among PBO, TNZ 2.5 mg, TNZ 2.5/5 mg and NSAID groups, incidences of an adjudicated outcome of normal progression of OA were similar (84.6–93.3%; lower with TNZ 5 mg [73.0%]). The TNZ 5 mg group had the highest percentage of pts with a TJR with an adjudicated outcome of RPOA1, RPOA2 or SIF (Table 1). Subgroup analyses did not reveal any associations between BL demographic or clinical characteristics and the incidence of TJR (Table 3).
Conclusion: Pts treated with TNZ 5 mg had a greater incidence of TJR than those receiving TNZ 2.5 mg, PBO or NSAID. The occurrence of TJR in the index joint in pts receiving TNZ 2.5 mg was similar to those receiving PBO or NSAID. Across all treatment groups, the majority of TJRs were not associated with an adjudicated joint safety event but rather normal progression of OA and occurred in a joint with structural evidence of OA.
Funded by Pfizer and Eli Lilly.
To cite this abstract in AMA style:
Carrino J, McAlindon T, Vignon E, Brown M, Burr A, Fountaine R, Pixton G, Viktrup L, West C, Verburg K. Total Joint Replacements in Three Phase 3 Studies of Tanezumab in Patients with Osteoarthritis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/total-joint-replacements-in-three-phase-3-studies-of-tanezumab-in-patients-with-osteoarthritis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/total-joint-replacements-in-three-phase-3-studies-of-tanezumab-in-patients-with-osteoarthritis/