Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Herpes zoster (HZ) is common in the elderly, with a lifetime risk of 25%. The primary risk factors for HZ are advanced age and immunosuppression. The aim is to describe the safety of recombinant zoster vaccine in patients with inflammatory rheumatic and musculoskeletal diseases (RMD)
Methods: Adult patients with rheumatoid arthritis (RA), axial spondyloarthritis (axSpA), and giant cell arteritis (GCA) were prospectively enrolled in this ongoing study. Data on demographics, vaccination, RMD diagnosis, disease activity, immunosuppressive treatments, flares, and zoster breakthrough infections were collected at months 0, 2, 3, 6 and 12. Safety assessments were performed at 2, 3, 6, and 12 months. A flare was defined as change in ASDAS ≥ 0.9 for axSpA, change in DAS-28 >1.2 for RA, or clinical signs for GCA and/or CRP ≥ 0.5 mg/dl and/or ≥30 mm. Descriptive analyses were performed
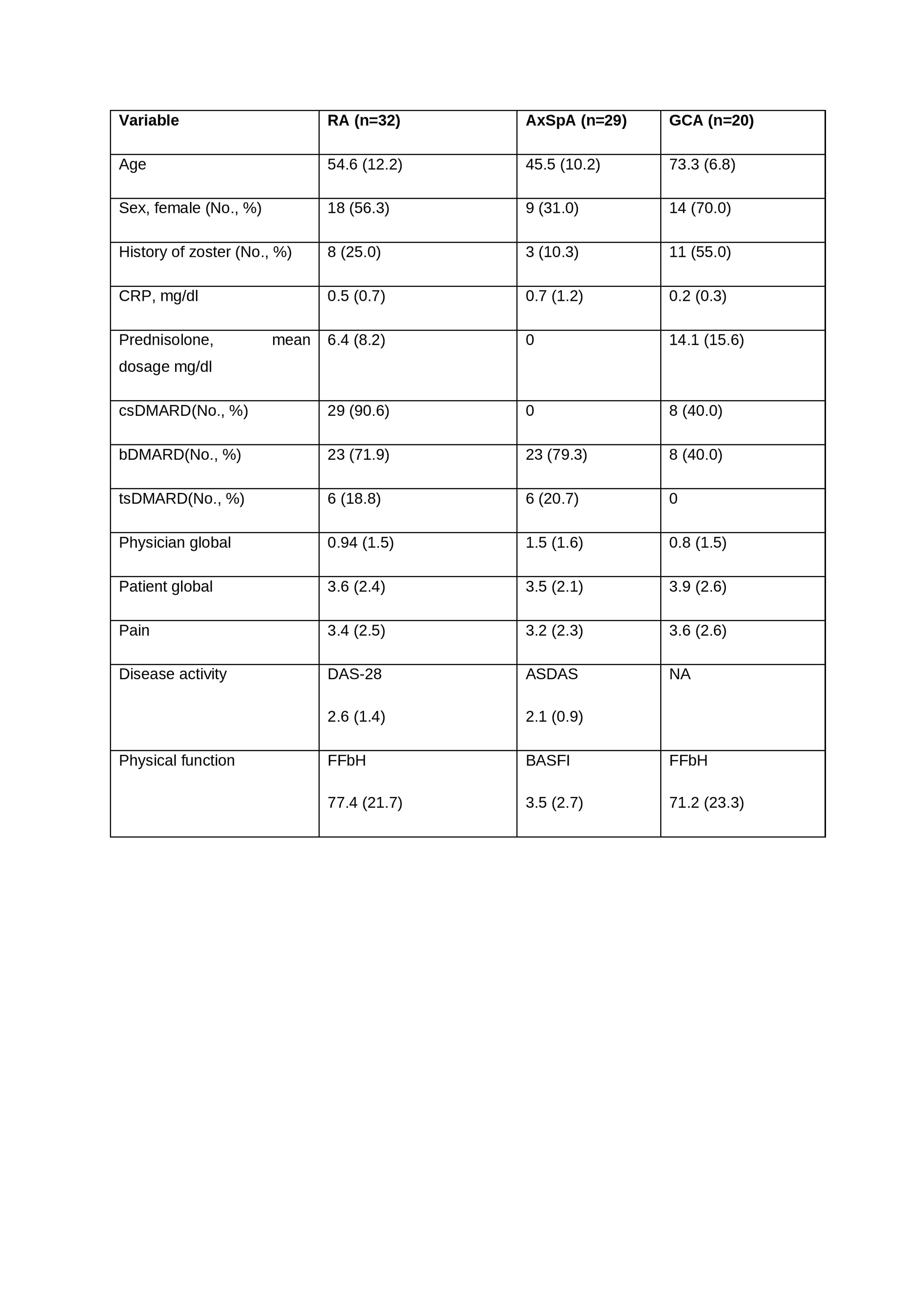
Results: 81 patients were enrolled, of whom 21 (25.9%) had a history of HZ (Table 1). All patients received RZV at month 0 and 66 patients at month 2. Safety assessments in 66, 56, 48, and 5 patients at month 2, 3, 6 and 12, respectively. A total of 87, 68, 15, 8 AEs were reported in 53, 37, 13, and 6 patients, respectively. Localized AEs (n=97 (67.0%)) were more common than generalized AEs (n=48 (33.1%)). Pain at the injection site (55 (30.9%)) was the most common AE, followed by fatigue (20 (11.2%)), musculoskeletal pain (19 (10.7%)), fever (14 (7.9%)), redness at the injection site (10 (5.6%)), and swelling at the injection site (7 (3.9%)). Serious adverse events (AE) were reported in 8 patients (3 RA, 5 GCA), none of which were vaccine-related. No patient reported an AE of special interest. 5, 4, 5 and 2 episodes of self-reported disease worsening were reported by patients at months 2, 3 and 6, respectively, 12 but none met predefined flare criteria. However, 3 patients (2 GCA, 1 RA) were hospitalized as a result. No episodes of HZ occurred during follow-up
Conclusion: Most patients tolerated RZV well with few reports of flare and serious AEs. The majority of AEs occurred within a few days of vaccination. These findings are reassuring for rheumatologists and potential vaccine recipients and support confidence in the safety of RZV in patients with RMD
To cite this abstract in AMA style:
Andreica I, Chierergo G, Reale S, Wilde B, Tsiami S, Kiefer D, Sewerin P, Kavruk H, Karagkiozidou D, Guminski B, Kribben A, Baraliakos X, Braun J, Kiltz U. Tolerability and Safety of Recombinant Zoster Vaccine in Patients with Inflammatory Rheumatic Musculoskeletal Diseases – A Prospective Longitudinal Study over 12 Months [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/tolerability-and-safety-of-recombinant-zoster-vaccine-in-patients-with-inflammatory-rheumatic-musculoskeletal-diseases-a-prospective-longitudinal-study-over-12-months/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tolerability-and-safety-of-recombinant-zoster-vaccine-in-patients-with-inflammatory-rheumatic-musculoskeletal-diseases-a-prospective-longitudinal-study-over-12-months/