Session Information
Date: Sunday, November 12, 2023
Title: (0283–0307) Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Approximately 1 in 4 people with RA have sarcopenia, defined as generalised loss of skeletal muscle (SKM) strength and mass, resulting in an increased risk of falls, fractures and mortality. Resistance exercise is the most effective treatment for sarcopenia and there is an unmet need for drugs that could augment or replace this, depending on an individual’s physical ability. Pooled data from studies investigating tofacitinib for the treatment of RA demonstrated small increases in serum creatinine (SCr) that were not associated with renal impairment and were inversely correlated with fall in CRP. SCr levels are influenced by SKM mass, raising the possibility that tofacitinib has an anti-sarcopenia effect. The Rheumatoid Arthritis and Muscle (RAMUS) study sought to test this hypothesis.
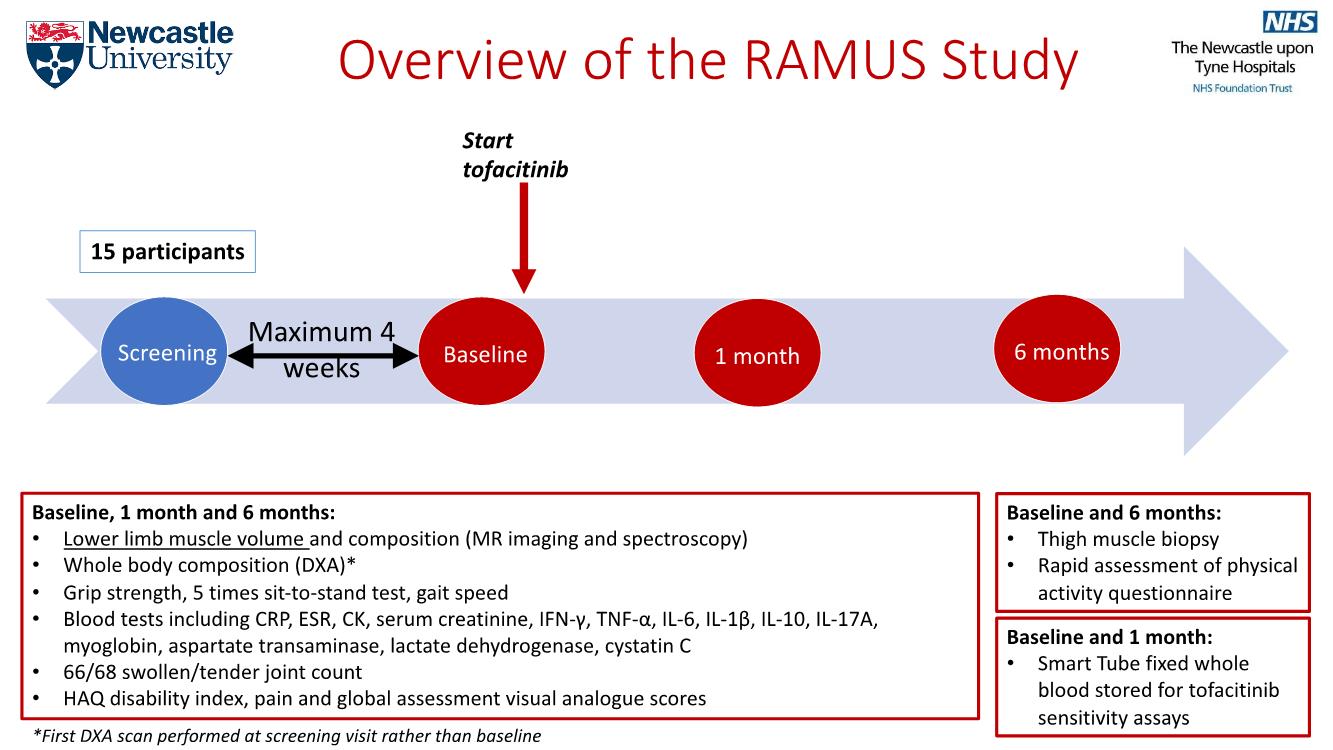
Methods: The RAMUS study is an observational, single-arm study of RA patients commenced on tofacitinib as part of routine care. Enrolment criteria included meeting the 2010 ACR/EULAR classification criteria for RA and >1 sarcopenia risk factor (low appendicular lean mass index, CRP > 5, low grip strength or prolonged sit-to-stand test time). Prior janus kinase (JAK) inhibitor or recent glucocorticoid treatment were not permitted. Assessments were at baseline, 1 and 6 months. The primary outcome was thigh and calf SKM volume determined by quantitative MRI. Whole body composition was measured by DXA. Study procedures are summarised in Figure 1.
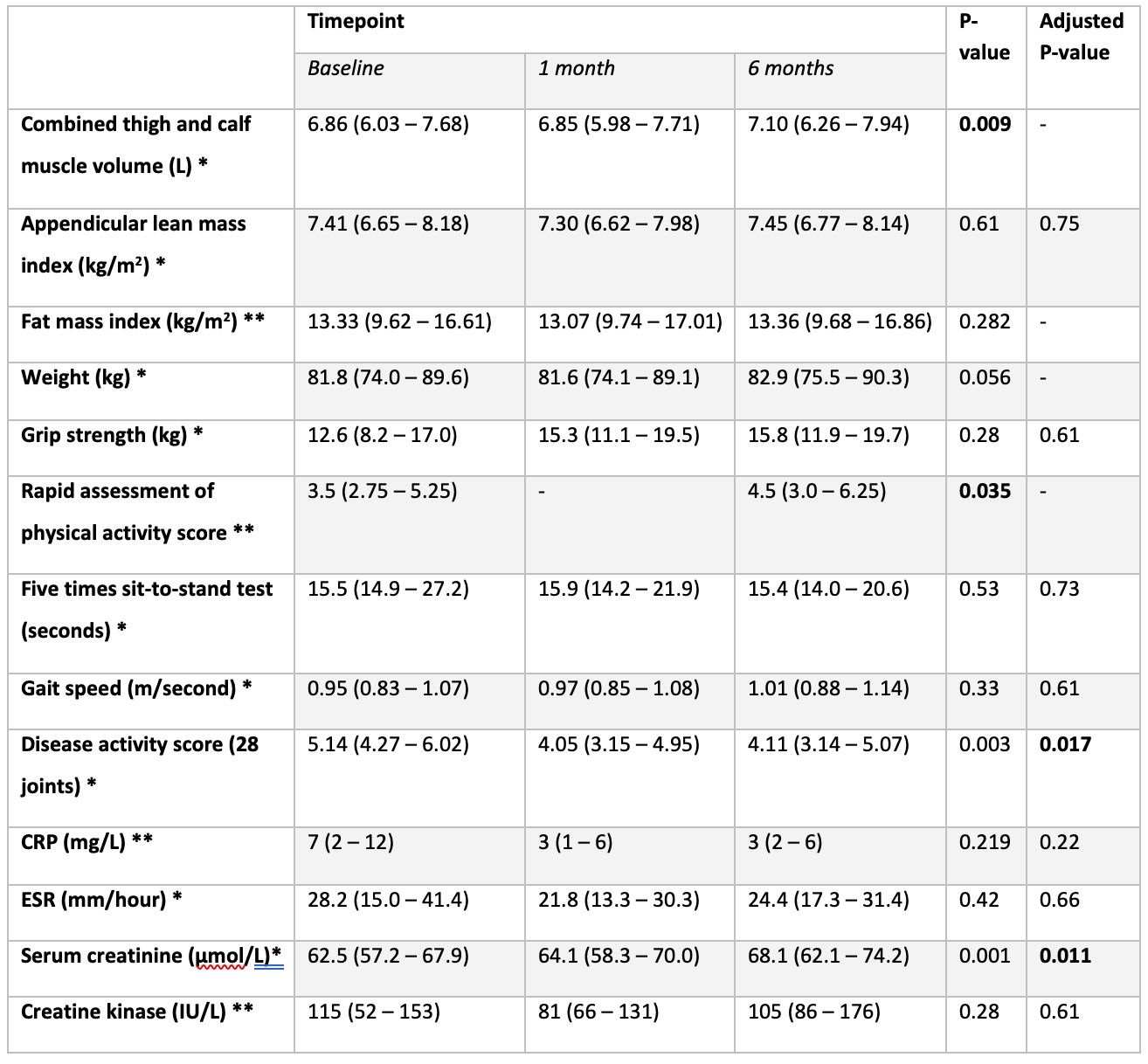
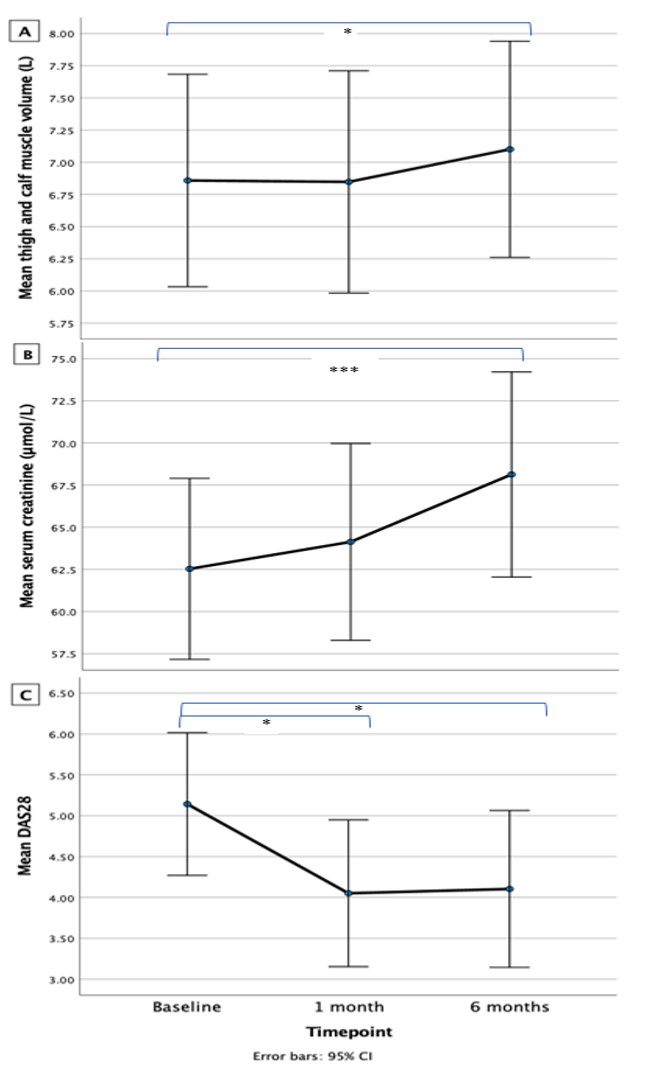
Results: 15 participants aged 41 – 73 years (mean 59) were enrolled (87% female). Median disease duration was 3.1 years (range 1.5 – 24.8) and 60% (n = 9) were seropositive (anti-CCP, RF or both). At baseline, 67% (n = 10) took methotrexate (no other conventional DMARDs were used), 87% (n = 13) were biologic naïve and median BMI was 31.8 kg/m2. All participants completed the study. 60% (n = 9) had >1 interruption of treatment ranging from 6 – 30 days. At 6 months, increases were observed in lower limb SKM volume (3.5% from baseline), SCr (9.0% from baseline) and self-reported physical activity; changes in disease activity manifested earlier, from 1 month (see Table 1 and Figure 2). 47% (n = 7) achieved a good (n = 3) or moderate (n = 4) EULAR response and 27% (n = 4) achieved ACR20. SKM volume increased significantly in the thigh but not in the calf. The muscle compartment fat fraction did not change. Grip strength, gait speed and fat mass index did not change. There was a trend towards increased weight. SKM volume correlated with grip strength (rs = 0.725, p = 0.003) at baseline. There was no correlation between SKM volume and SCr (either at baseline or in the difference at 6 months).
Conclusion: Treating RA with tofacitinib for 6 months was associated with increased SKM volume, increased SCr and increased self-reported physical activity. Possible mechanisms include a direct anabolic effect of JAK inhibition on SKM, reduced systemic inflammation, improved RA symptoms or a combination of the above. Analysis of SKM tissue samples from participants in the RAMUS study will provide mechanistic insight. These data merit further investigation in the form of a randomised trial to test whether the improvements are specific to tofacitinib and whether combining JAK inhibition with resistance exercise yields greater benefits.
To cite this abstract in AMA style:
Bennett J, Hollingsworth K, Pratt A, Egail M, Feeney C, Di Leo V, Taylor R, Dodds R, Anderson A, Sayer A, Isaacs J. Tofacitinib Treatment in Rheumatoid Arthritis Is Associated with Increased Lower Limb Muscle Volume: The Rheumatoid Arthritis and Muscle (RAMUS) Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/tofacitinib-treatment-in-rheumatoid-arthritis-is-associated-with-increased-lower-limb-muscle-volume-the-rheumatoid-arthritis-and-muscle-ramus-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tofacitinib-treatment-in-rheumatoid-arthritis-is-associated-with-increased-lower-limb-muscle-volume-the-rheumatoid-arthritis-and-muscle-ramus-study/